Ceruloplasmin and hephaestin jointly protect the exocrine pancreas against oxidative damage by facilitating iron efflux
- PMID: 29883959
- PMCID: PMC6007082
- DOI: 10.1016/j.redox.2018.05.013
Ceruloplasmin and hephaestin jointly protect the exocrine pancreas against oxidative damage by facilitating iron efflux
Abstract
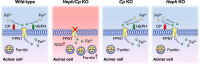
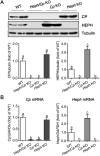
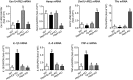
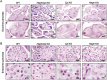
Little is known about the iron efflux from the pancreas, but it is likely that multicopper ferroxidases (MCFs) are involved in this process. We thus used hephaestin (Heph) and ceruloplasmin (Cp) single-knockout mice and Heph/Cp double-knockout mice to investigate the roles of MCFs in pancreatic iron homeostasis. We found that both HEPH and CP were expressed in the mouse pancreas, and that ablation of either MCF had limited effect on the pancreatic iron levels. However, ablation of both MCFs together led to extensive pancreatic iron deposition and severe oxidative damage. Perls' Prussian blue staining revealed that this iron deposition was predominantly in the exocrine pancreas, while the islets were spared. Consistent with these results, plasma lipase and trypsin were elevated in Heph/Cp knockout mice, indicating damage to the exocrine pancreas, while insulin secretion was not affected. These data indicate that HEPH and CP play mutually compensatory roles in facilitating iron efflux from the exocrine pancreas, and show that MCFs are able to protect the pancreas against iron-induced oxidative damage.
Keywords: Ceruloplasmin; Hephaestin; Iron efflux; Multicopper ferroxidase; Oxidative damage; Pancreas.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Hephaestin and ceruloplasmin facilitate iron metabolism in the mouse kidney.Sci Rep. 2016 Dec 19;6:39470. doi: 10.1038/srep39470. Sci Rep. 2016. PMID: 27991585 Free PMC article.
-
Ablation of hephaestin and ceruloplasmin results in iron accumulation in adipocytes and type 2 diabetes.FEBS Lett. 2018 Feb;592(3):394-401. doi: 10.1002/1873-3468.12978. Epub 2018 Jan 31. FEBS Lett. 2018. PMID: 29355933
-
Severe Iron Metabolism Defects in Mice With Double Knockout of the Multicopper Ferroxidases Hephaestin and Ceruloplasmin.Cell Mol Gastroenterol Hepatol. 2018 Jun 23;6(4):405-427. doi: 10.1016/j.jcmgh.2018.06.006. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30182051 Free PMC article.
-
The biology of mammalian multi-copper ferroxidases.Biometals. 2023 Apr;36(2):263-281. doi: 10.1007/s10534-022-00370-z. Epub 2022 Feb 15. Biometals. 2023. PMID: 35167013 Free PMC article. Review.
-
[Ceruloplasmin, hephaestin and zyklopen: the three multicopper oxidases important for human iron metabolism].Postepy Hig Med Dosw (Online). 2014;68:912-24. doi: 10.5604/17322693.1111136. Postepy Hig Med Dosw (Online). 2014. PMID: 24988611 Review. Polish.
Cited by
-
Iron-Storage Disorder Presenting as Chronic Diarrhea.Cureus. 2021 Oct 18;13(10):e18864. doi: 10.7759/cureus.18864. eCollection 2021 Oct. Cureus. 2021. PMID: 34804717 Free PMC article.
-
Glucose supplementation improves intestinal amino acid transport and muscle amino acid pool in pigs during chronic cold exposure.Anim Nutr. 2022 Dec 9;12:360-374. doi: 10.1016/j.aninu.2022.10.009. eCollection 2023 Mar. Anim Nutr. 2022. PMID: 36788930 Free PMC article.
-
Aceruloplasminemia: Waiting for an Efficient Therapy.Front Neurosci. 2018 Dec 4;12:903. doi: 10.3389/fnins.2018.00903. eCollection 2018. Front Neurosci. 2018. PMID: 30568573 Free PMC article.
-
A role for ceruloplasmin in the control of human glioblastoma cell responses to radiation.BMC Cancer. 2022 Aug 2;22(1):843. doi: 10.1186/s12885-022-09808-6. BMC Cancer. 2022. PMID: 35918659 Free PMC article.
-
Ceruloplasmin administration in the preclinical mouse model of aceruloplasminemia reveals a sex-related variation in biodistribution.Commun Biol. 2025 Feb 19;8(1):264. doi: 10.1038/s42003-025-07714-8. Commun Biol. 2025. PMID: 39972187 Free PMC article.
References
-
- Puntarulo S. Iron, oxidative stress and human health. Mol. Asp. Med. 2005;26:299–312. - PubMed
-
- Andrews N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999;341:1986–1995. - PubMed
-
- Lieu P.T., Heiskala M., Peterson P.A., Yang Y. The roles of iron in health and disease. Mol. Asp. Med. 2001;22:1–87. - PubMed
-
- Chen H., Attieh Z.K., Syed B.A., Kuo Y.M., Stevens V., Fuqua B.K., Andersen H.S., Naylor C.E., Evans R.W., Gambling L., Danzeisen R., Bacouri-Haidar M., Usta J., Vulpe C.D., McArdle H.J. Identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells. J. Nutr. 2010;140:1728–1735. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

