A PSTOL-like gene, TaPSTOL, controls a number of agronomically important traits in wheat
- PMID: 29884124
- PMCID: PMC5994007
- DOI: 10.1186/s12870-018-1331-4
A PSTOL-like gene, TaPSTOL, controls a number of agronomically important traits in wheat
Abstract
Background: Phosphorus (P) is an essential macronutrient for plant growth, and is required in large quantities by elite varieties of crops to maintain yields. Approximately 70% of global cultivated land suffers from P deficiency, and it has recently been estimated that worldwide P resources will be exhausted by the end of this century, increasing the demand for crops more efficient in their P usage. A greater understanding of how plants are able to maintain yield with lower P inputs is, therefore, highly desirable to both breeders and farmers. Here, we clone the wheat (Triticum aestivum L.) homologue of the rice PSTOL gene (OsPSTOL), and characterize its role in phosphate nutrition plus other agronomically important traits.
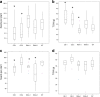
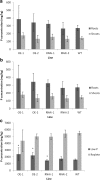
Results: TaPSTOL is a single copy gene located on the short arm of chromosome 5A, encoding a putative kinase protein, and shares a high level of sequence similarity to OsPSTOL. We re-sequenced TaPSTOL from 24 different wheat accessions and (3) three T. durum varieties. No sequence differences were detected in 26 of the accessions, whereas two indels were identified in the promoter region of one of the durum wheats. We characterised the expression of TaPSTOL under different P concentrations and demonstrated that the promoter was induced in root tips and hairs under P limiting conditions. Overexpression and RNAi silencing of TaPSTOL in transgenic wheat lines showed that there was a significant effect upon root biomass, flowering time independent of P treatment, tiller number and seed yield, correlating with the expression of TaPSTOL. However this did not increase PUE as elevated P concentration in the grain did not correspond to increased yields.
Conclusions: Manipulation of TaPSTOL expression in wheat shows it is responsible for many of the previously described phenotypic advantages as OsPSTOL except yield. Furthermore, we show TaPSTOL contributes to additional agronomically important traits including flowering time and grain size. Analysis of TaPSTOL sequences from a broad selection of wheat varieties, encompassing 91% of the genetic diversity in UK bread wheat, showed that there is very little genetic variation in this gene, which would suggest that this locus may have been under high selection pressure.
Keywords: Flowering time; P; PSTOL; PUE; Phosphate; Seed number; Seed size; Wheat.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
-
- Kirkby EA, Johnston A. The ecophysiology of plant-phosphorus interactions. Netherlands: Springer; 2008.
-
- Hinsinger P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil. 2001;237:173–195. doi: 10.1023/A:1013351617532. - DOI
-
- Cakmak I. Plant nutrition research: Priorities to meet human needs for food in sustainable ways. Prog Plant Nutr Plenary Lect XIV Int Plant Nutr Colloq. Dordrecht: Springer Netherlands; 2002. pp. 3–24.
-
- Lynch JP, Brown KM. Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant Soil. 2001;237:225–37.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

