Insights into the ecology, evolution, and metabolism of the widespread Woesearchaeotal lineages
- PMID: 29884244
- PMCID: PMC5994134
- DOI: 10.1186/s40168-018-0488-2
Insights into the ecology, evolution, and metabolism of the widespread Woesearchaeotal lineages
Abstract
Background: As a recently discovered member of the DPANN superphylum, Woesearchaeota account for a wide diversity of 16S rRNA gene sequences, but their ecology, evolution, and metabolism remain largely unknown.
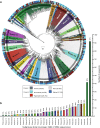
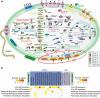
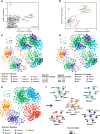
Results: Here, we assembled 133 global clone libraries/studies and 19 publicly available genomes to profile these patterns for Woesearchaeota. Phylogenetic analysis shows a high diversity with 26 proposed subgroups for this recently discovered archaeal phylum, which are widely distributed in different biotopes but primarily in inland anoxic environments. Ecological patterns analysis and ancestor state reconstruction for specific subgroups reveal that oxic status of the environments is the key factor driving the distribution and evolutionary diversity of Woesearchaeota. A selective distribution to different biotopes and an adaptive colonization from anoxic to oxic environments can be proposed and supported by evidence of the presence of ferredoxin-dependent pathways in the genomes only from anoxic biotopes but not from oxic biotopes. Metabolic reconstructions support an anaerobic heterotrophic lifestyle with conspicuous metabolic deficiencies, suggesting the requirement for metabolic complementarity with other microbes. Both lineage abundance distribution and co-occurrence network analyses across diverse biotopes confirmed metabolic complementation and revealed a potential syntrophic relationship between Woesearchaeota and methanogens, which is supported by metabolic modeling. If correct, Woesearchaeota may impact methanogenesis in inland ecosystems.
Conclusions: The findings provide an ecological and evolutionary framework for Woesearchaeota at a global scale and indicate their potential ecological roles, especially in methanogenesis.
Keywords: Ecology; Evolution; Metabolism; Methanogen; Subgroup; Woesearchaeota.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

