Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression
- PMID: 29884413
- PMCID: PMC6563923
- DOI: 10.1016/j.oraloncology.2018.04.008
Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression
Abstract
Objectives: We report 2-year results from CheckMate 141 to establish the long-term efficacy and safety profile of nivolumab and outcomes by tumor PD-L1 expression in patients with recurrent or metastatic (R/M),platinum-refractory squamous cell carcinoma of the head and neck (SCCHN).
Methods: Patients with R/M SCCHN with tumor progression/recurrence within 6 months of platinum therapy were randomized 2:1 to nivolumab 3 mg/kg every 2 weeks or investigator's choice (IC). Primary endpoint: overall survival (OS). Data cutoff: September 2017.
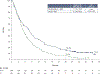
Results: With 24.2 months' minimum follow-up, nivolumab (n = 240) continued to improve OS vs IC (n = 121), hazard ratio (HR) = 0.68 (95% CI 0.54-0.86). Nivolumab nearly tripled the estimated 24-month OS rate (16.9%) vs IC (6.0%), and demonstrated OS benefit across patients with tumor PD-L1 expression ≥1% (HR [95% CI] = 0.55 [0.39-0.78]) and < 1% (HR [95% CI] = 0.73 [0.49-1.09]), and regardless of tumor HPV status. Estimated OS rates at 18, 24, and 30 months with nivolumab were consistent irrespective of PD-L1 expression (<1%/≥1%). In the nivolumab arm, there were no observed differences in baseline characteristics or safety profile between long-term survivors and the overall population. Grade 3-4 treatment-related adverse event rates were 15.3% and 36.9% for nivolumab and IC, respectively.
Conclusion: Nivolumab significantly improved OS at the primary analysis and demonstrated prolonged OS benefit vs IC and maintenance of a manageable and consistent safety profile with 2-year follow-up. OS benefit was observed with nivolumab irrespective of PD-L1 expression and HPV status. (Clinicaltrials.gov: NCT02105636).
Keywords: CD274 protein, human (PD-L1 Protein, Human); Carcinoma, squamous cell of head and neck; Clinical Trial, Phase III; Head and Neck Neoplasms; Immunotherapy; Nivolumab; Papillomaviridae (HPV, Human Papillomavirus Viruses); Programmed Cell Death 1 Receptor; Survival Analysis; Survivors (Long-term Survivors).
Copyright © 2018. Published by Elsevier Ltd.
Figures
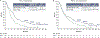
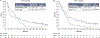
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. - PubMed
-
- Bernier J Drug Insight: cetuximab in the treatment of recurrent and metastatic squamous cell carcinoma of the head and neck. Nat Clin Pract Oncol 2008;5:705–13. - PubMed
-
- Saloura V, Cohen EE, Licitra L, Billan S, Dinis J, Lisby S, et al. An open-label singlearm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 2014;73:1227–39. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

