m6A RNA Degradation Products Are Catabolized by an Evolutionarily Conserved N6-Methyl-AMP Deaminase in Plant and Mammalian Cells
- PMID: 29884623
- PMCID: PMC6096584
- DOI: 10.1105/tpc.18.00236
m6A RNA Degradation Products Are Catabolized by an Evolutionarily Conserved N6-Methyl-AMP Deaminase in Plant and Mammalian Cells
Abstract
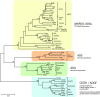
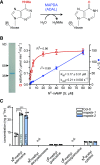
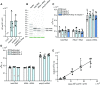
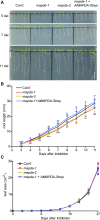
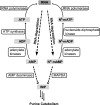
N6-methylated adenine (m6A) is the most frequent posttranscriptional modification in eukaryotic mRNA. Turnover of RNA generates N6-methylated AMP (N6-mAMP), which has an unclear metabolic fate. We show that Arabidopsis thaliana and human cells require an N6-mAMP deaminase (ADAL, renamed MAPDA) to catabolize N6-mAMP to inosine monophosphate in vivo by hydrolytically removing the aminomethyl group. A phylogenetic, structural, and biochemical analysis revealed that many fungi partially or fully lack MAPDA, which coincides with a minor role of N6A-RNA methylation in these organisms. MAPDA likely protects RNA from m6A misincorporation. This is required because eukaryotic RNA polymerase can use N6-mATP as a substrate. Upon abrogation of MAPDA, root growth is slightly reduced, and the N6-methyladenosine, N6-mAMP, and N6-mATP concentrations are increased in Arabidopsis. Although this will potentially lead to m6A misincorporation into RNA, we show that the frequency is too low to be reliably detected in vivo. Since N6-mAMP was severalfold more abundant than N6-mATP in MAPDA mutants, we speculate that additional molecular filters suppress the generation of N6-mATP. Enzyme kinetic data indicate that adenylate kinases represent such filters being highly selective for AMP versus N6-mAMP phosphorylation. We conclude that a multilayer molecular protection system is in place preventing N6-mAMP accumulation and salvage.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures





Comment in
-
The Story Continues: Following the Fate of m6A Marks in the Eukaryotic Transcriptome.Plant Cell. 2018 Jul;30(7):1385-1386. doi: 10.1105/tpc.18.00456. Epub 2018 Jun 14. Plant Cell. 2018. PMID: 29903727 Free PMC article. No abstract available.
References
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

