Metformin induces FOXO3-dependent fetal hemoglobin production in human primary erythroid cells
- PMID: 29884740
- PMCID: PMC6053951
- DOI: 10.1182/blood-2017-11-814335
Metformin induces FOXO3-dependent fetal hemoglobin production in human primary erythroid cells
Abstract
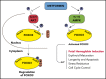
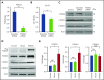
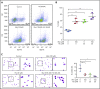
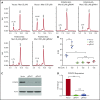
Induction of red blood cell (RBC) fetal hemoglobin (HbF; α2γ2) ameliorates the pathophysiology of sickle cell disease (SCD) by reducing the concentration of sickle hemoglobin (HbS; α2βS2) to inhibit its polymerization. Hydroxyurea (HU), the only US Food and Drug Administration (FDA)-approved drug for SCD, acts in part by inducing HbF; however, it is not fully effective, reflecting the need for new therapies. Whole-exome sequence analysis of rare genetic variants in SCD patients identified FOXO3 as a candidate regulator of RBC HbF. We validated these genomic findings through loss- and gain-of-function studies in normal human CD34+ hematopoietic stem and progenitor cells induced to undergo erythroid differentiation. FOXO3 gene silencing reduced γ-globin RNA levels and HbF levels in erythroblasts, whereas overexpression of FOXO3 produced the opposite effect. Moreover, treatment of primary CD34+ cell-derived erythroid cultures with metformin, an FDA-approved drug known to enhance FOXO3 activity in nonerythroid cells, caused dose-related FOXO3-dependent increases in the percentage of HbF protein and the fraction of HbF-immunostaining cells (F cells). Combined HU and metformin treatment induced HbF additively and reversed the arrest in erythroid maturation caused by HU treatment alone. HbF induction by metformin in erythroid precursors was dependent on FOXO3 expression and did not alter expression of BCL11A, MYB, or KLF1. Collectively, our data implicate FOXO3 as a positive regulator of γ-globin expression and identify metformin as a potential therapeutic agent for SCD.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
-
- Odenheimer DJ, Sarnaik SA, Whitten CF, Rucknagel DL, Sing CF. The relationship between fetal hemoglobin and disease severity in children with sickle cell anemia. Am J Med Genet. 1987;27(3):525-535. - PubMed
-
- Charache S, Barton FB, Moore RD, et al. ; The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. Medicine (Baltimore). 1996;75(6):300-326. - PubMed
-
- Watson J. The significance of the paucity of sickle cells in newborn Negro infants. Am J Med Sci. 1948;215(4):419-423. - PubMed
-
- Steinberg MH, McCarthy WF, Castro O, et al. ; Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia and MSH Patients’ Follow-Up. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol. 2010;85(6):403-408. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

