Novel hyperoxidation resistance motifs in 2-Cys peroxiredoxins
- PMID: 29884768
- PMCID: PMC6066324
- DOI: 10.1074/jbc.RA117.001690
Novel hyperoxidation resistance motifs in 2-Cys peroxiredoxins
Abstract
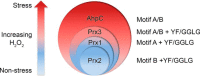
2-Cys peroxiredoxins (Prxs) modulate hydrogen peroxide (H2O2)-mediated cell signaling. At high H2O2 levels, eukaryotic Prxs can be inactivated by hyperoxidation and are classified as sensitive Prxs. In contrast, prokaryotic Prxs are categorized as being resistant to hyperoxidation and lack the GGLG and C-terminal YF motifs present in the sensitive Prxs. Additional molecular determinants that account for the subtle differences in the susceptibility to hyperoxidation remain to be identified. A comparison of a new, 2.15-Å-resolution crystal structure of Prx2 in the oxidized, disulfide-bonded state with the hyperoxidized structure of Prx2 and Prx1 in complex with sulfiredoxin revealed three structural regions that rearrange during catalysis. With these regions in hand, focused sequence analyses were performed comparing sensitive and resistant Prx groups. From this combinatorial approach, we discovered two novel hyperoxidation resistance motifs, motifs A and B, which were validated using mutagenesis of sensitive human Prxs and resistant Salmonella enterica serovar Typhimurium AhpC. Introduction and removal of these motifs, respectively, resulted in drastic changes in the sensitivity to hyperoxidation with Prx1 becoming 100-fold more resistant to hyperoxidation and AhpC becoming 800-fold more sensitive to hyperoxidation. The increased sensitivity of the latter AhpC variant was also confirmed in vivo These results support the function of motifs A and B as primary drivers for tuning the sensitivity of Prxs to different levels of H2O2, thus enabling the initiation of variable signaling or antioxidant responses in cells.
Keywords: Cys sulfinic acid; X-ray crystallography; cell signaling; enzyme kinetics; oxidation-reduction (redox); peroxiredoxin; protein oxidation; redox biology; structural biology.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Rabilloud T., Heller M., Gasnier F., Luche S., Rey C., Aebersold R., Benahmed M., Louisot P., and Lunardi J. (2002) Proteomics analysis of cellular response to oxidative stress: evidence for in vivo overoxidation of peroxiredoxins at their active site. J. Biol. Chem. 277, 19396–19401 10.1074/jbc.M106585200 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

