Ni(II)-catalyzed asymmetric alkenylations of ketimines
- PMID: 29884893
- PMCID: PMC5993804
- DOI: 10.1038/s41467-018-04645-3
Ni(II)-catalyzed asymmetric alkenylations of ketimines
Abstract
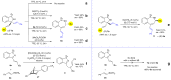
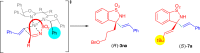
Chiral allylic amines are not only present in many bioactive compounds, but can also be readily transformed to other chiral amines. Therefore, the asymmetric synthesis of chiral allylic amines is highly desired. Herein, we report two types of Ni(II)-catalyzed asymmetric alkenylation of cyclic ketimines for the preparation of chiral allylic amines. When ketimines bear alkyl or alkoxycarbonyl groups, the alkenylation gives five- and six-membered cyclic α-tertiary allylic amine products with excellent yields and enantioselectivities under mild reaction conditions. A variety of ketimines can be used and the method tolerates some variation in alkenylboronic acid scope. Furthermore, with alkenyl five-membered ketimine substrates, an alkenylation/rearrangement reaction occurs, providing seven-membered chiral sulfamide products bearing a conjugated diene skeleton with excellent yields and enantioselectivities. Mechanistic studies reveal that the ring expansion step is a stereospecific site-selective process, which can be catalyzed by acid (Lewis acid or Brønsted acid).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Bullock, R. M. (Ed) Catalysis Without Precious Metals (Wiley-VCH, Weinheim, 2010).
-
- Zhu SF, Zhou QL. Iron-catalyzed transformations of diazo compounds. Natl. Sci. Rev. 2014;1:580–603. doi: 10.1093/nsr/nwu019. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

