Sialylated Receptor Setting Influences Mycoplasma pneumoniae Attachment and Gliding Motility
- PMID: 29885004
- PMCID: PMC6185809
- DOI: 10.1111/mmi.13997
Sialylated Receptor Setting Influences Mycoplasma pneumoniae Attachment and Gliding Motility
Abstract
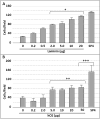
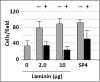
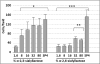
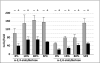
Mycoplasma pneumoniae is a common cause of human respiratory tract infections, including bronchitis and atypical pneumonia. M. pneumoniae binds glycoprotein receptors having terminal sialic acid residues via the P1 adhesin protein. Here, we explored the impact of sialic acid presentation on M. pneumoniae adherence and gliding on surfaces coated with sialylated glycoproteins, or chemically functionalized with α-2,3- and α-2,6-sialyllactose ligated individually or in combination to a polymer scaffold in precisely controlled densities. In both models, gliding required a higher receptor density threshold than adherence, and receptor density influenced gliding frequency but not gliding speed. However, very high densities of α-2,3-sialyllactose actually reduced gliding frequency over peak levels observed at lower densities. Both α-2,3- and α-2,6-sialyllactose supported M. pneumoniae adherence, but gliding was only observed on the former. Finally, gliding on α-2,3-sialyllactose was inhibited on surfaces also conjugated with α-2,6-sialyllactose, suggesting that both moieties bind P1 despite the inability of the latter to support gliding. Our results indicate that the nature and density of host receptor moieties profoundly influences M. pneumoniae gliding, which could affect pathogenesis and infection outcome. Furthermore, precise functionalization of polymer scaffolds shows great promise for further analysis of sialic acid presentation and M. pneumoniae adherence and gliding.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
We declare no competing interests.
Figures




Similar articles
-
Distinct Mycoplasma pneumoniae Interactions with Sulfated and Sialylated Receptors.Infect Immun. 2020 Oct 19;88(11):e00392-20. doi: 10.1128/IAI.00392-20. Print 2020 Oct 19. Infect Immun. 2020. PMID: 32839185 Free PMC article.
-
Behaviors and Energy Source of Mycoplasma gallisepticum Gliding.J Bacteriol. 2019 Sep 6;201(19):e00397-19. doi: 10.1128/JB.00397-19. Print 2019 Oct 1. J Bacteriol. 2019. PMID: 31308069 Free PMC article.
-
Adhesion and biofilm formation of Mycoplasma pneumoniae on an abiotic surface.Arch Microbiol. 2011 Nov;193(11):833-6. doi: 10.1007/s00203-011-0749-y. Epub 2011 Aug 31. Arch Microbiol. 2011. PMID: 21879294
-
Mycoplasma pneumoniae cytadherence: unravelling the tie that binds.Mol Microbiol. 1996 Apr;20(2):247-53. doi: 10.1111/j.1365-2958.1996.tb02613.x. Mol Microbiol. 1996. PMID: 8733224 Review.
-
Structure, function, and assembly of the terminal organelle of Mycoplasma pneumoniae.FEMS Microbiol Lett. 2001 Apr 20;198(1):1-7. doi: 10.1111/j.1574-6968.2001.tb10610.x. FEMS Microbiol Lett. 2001. PMID: 11325545 Review.
Cited by
-
Insight into the Pathogenic Mechanism of Mycoplasma pneumoniae.Curr Microbiol. 2022 Dec 2;80(1):14. doi: 10.1007/s00284-022-03103-0. Curr Microbiol. 2022. PMID: 36459213 Free PMC article. Review.
-
Unraveling the role of distinct cytoskeletal motility structures in Mycoplasma pneumoniae relatives.BMC Microbiol. 2025 Aug 29;25(1):562. doi: 10.1186/s12866-025-04320-w. BMC Microbiol. 2025. PMID: 40883708 Free PMC article. Review.
-
Mycoplasma pneumoniae Infections: Pathogenesis and Vaccine Development.Pathogens. 2021 Jan 25;10(2):119. doi: 10.3390/pathogens10020119. Pathogens. 2021. PMID: 33503845 Free PMC article. Review.
-
Respiratory microbiome and metabolome features associate disease severity and the need for doxycycline treatment in children with macrolide-resistant Mycoplasma pneumoniae-mediated pneumonia.Front Cell Infect Microbiol. 2025 Jul 28;15:1537182. doi: 10.3389/fcimb.2025.1537182. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40792100 Free PMC article.
-
New Insights into the Host-Pathogen Interaction of Mycoplasma gallisepticum and Avian Metapneumovirus in Tracheal Organ Cultures of Chicken.Microorganisms. 2021 Nov 22;9(11):2407. doi: 10.3390/microorganisms9112407. Microorganisms. 2021. PMID: 34835532 Free PMC article.
References
-
- Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32:956–973. - PubMed
-
- Baseman JB, Morrison-Plummer J, Drouillard D, Puleo-Scheppke B, Tryon VV, Holt SC. Identification of a 32-kilodalton protein of Mycoplasma pneumoniae associated with hemadsorption. Isr J Med Sci. 1987;23:474–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

