Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study
- PMID: 29886024
- PMCID: PMC6626762
- DOI: 10.1016/S2213-2600(18)30202-9
Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study
Erratum in
-
Corrections.Lancet Respir Med. 2018 Jul;6(7):e35. doi: 10.1016/S2213-2600(18)30244-3. Lancet Respir Med. 2018. PMID: 29976450 No abstract available.
-
Correction to Lancet Respir Med 2018; 6: 545-53.Lancet Respir Med. 2019 Apr;7(4):e15. doi: 10.1016/S2213-2600(19)30094-3. Lancet Respir Med. 2019. PMID: 30926052 No abstract available.
Abstract
Background: Ivacaftor is generally safe and effective in patients aged 2 years and older who have cystic fibrosis and specific CFTR mutations. We assessed its use in children aged 12 to <24 months.
Methods: The ARRIVAL study is a phase 3, single-arm, two-part, multicentre study. Eligible children were aged 12 to <24 months at enrolment and had a confirmed diagnosis of cystic fibrosis and a CFTR gating mutation on at least one allele and could participate in one or both parts of the study. Children received 50 mg (bodyweight 7 to <14 kg) or 75 mg (bodyweight ≥14 to <25 kg) ivacaftor orally every 12 h. In study part A, children received ivacaftor for 3 days plus one morning. In study part B, children received 24 weeks of treatment. Children were enrolled into part A at seven sites in Australia (one site), the UK (one), and the USA (five) and into part B at 13 sites in Australia (two sites), Canada (one), the UK (three), and the USA (seven). Primary endpoints were pharmacokinetics (part A) and safety (parts A and B) in children who received at least one dose of ivacaftor. Secondary endpoints in part B were pharmacokinetics in children who received at least one dose of ivacaftor and absolute change from baseline in sweat chloride concentration. We also explored changes in growth parameters and markers of pancreatic function. This study is registered with ClinicalTrials.gov, number NCT02725567.
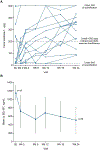
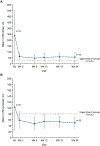
Findings: Children aged 12 to <24 months were enrolled between Aug 25, 2016, and Nov 1, 2017. Seven children were enrolled in part A, of whom five received 50 mg and two received 75 mg ivacaftor. All completed treatment. Of 19 children enrolled in part B, including one from part A, all received 50 mg ivacaftor and 18 completed treatment (one withdrew because of difficulty with blood draws). All children received at least one dose of ivacaftor. Pharmacokinetics indicated exposure was similar to that in children aged 2 to <6 years and adults. No children discontinued because of adverse events or safety findings. In part A, three (43%) of seven children had treatment-emergent adverse events, all of which were mild and deemed not to be or unlikely to be related to ivacaftor. By 24 weeks in part B, treatment-emergent adverse events had been reported in 18 (95%) of 19 children, of which most were mild or moderate and the most frequent was cough (14 [74%] children). Two children in part B had four serious adverse events: one had constipation (possibly related to ivacaftor), distal intestinal obstruction syndrome, and eczema herpeticum, and one had persistent cough, all needing hospital admission. In five (28%) of 18 children aspartate or alanine aminotransferase concentrations rose to more than three times the upper limit of normal (to more than eight times in two children with concurrent infections). At week 24, the mean absolute change from baseline in sweat chloride concentration was -73·5 (SD 17·5) mmol/L. Growth parameters for age were normal at baseline and at week 24. At week 24, concentrations of faecal elastase-1 had increased and concentrations of immunoreactive trypsinogen had decreased from baseline. Mean serum lipase and amylase were raised at baseline and rapidly decreased after treatment was started.
Interpretation: Ivacaftor was generally safe and well tolerated in children aged 12 to <24 months for up to 24 weeks and was associated with rapid and sustained reductions in sweat chloride concentrations. Improvements in biomarkers of pancreatic function suggest that ivacaftor preserves exocrine pancreatic function if started early. The study is continuing in infants younger than 12 months.
Funding: Vertex Pharmaceuticals Incorporated.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Treatment of cystic fibrosis in infants.Lancet Respir Med. 2018 Jul;6(7):483-484. doi: 10.1016/S2213-2600(18)30203-0. Epub 2018 Jun 7. Lancet Respir Med. 2018. PMID: 29886025 No abstract available.
References
-
- O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009; 373: 1891–904. - PubMed
-
- Stick SM, Brennan S, Murray C, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr 2009; 155: 623–8. - PubMed
-
- Long FR, Williams RS, Castile RG. Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr 2004; 144: 154–61. - PubMed
-
- Mott LS, Park J, Murray CP, et al. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax 2012; 67: 509–16. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

