The Lateral Habenula Directs Coping Styles Under Conditions of Stress via Recruitment of the Endocannabinoid System
- PMID: 29887035
- PMCID: PMC6162143
- DOI: 10.1016/j.biopsych.2018.04.018
The Lateral Habenula Directs Coping Styles Under Conditions of Stress via Recruitment of the Endocannabinoid System
Abstract
Background: The ability to effectively cope with stress is a critical determinant of disease susceptibility. The lateral habenula (LHb) and the endocannabinoid (ECB) system have independently been shown to be involved in the selection of stress coping strategies, yet the role of ECB signaling in the LHb remains unknown.
Methods: Using a battery of complementary techniques in rats, we examined the localization of type-1 cannabinoid receptors (CB1Rs) and assessed the behavioral and neuroendocrine effects of intra-LHb CB1R manipulations. We further tested the extent to which the ECB system in the LHb is impacted following chronic unpredictable stress or social defeat stress, and whether manipulation of LHb CB1Rs can bias coping strategies in rats with a history of chronic stress.
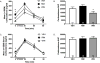
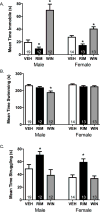
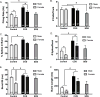
Results: Electron microscopy studies revealed CB1R expression on presynaptic axon terminals, postsynaptic membranes, mitochondria, and glial processes in the rat LHb. In vivo microdialysis experiments indicated that acute stress increased the amount of 2-arachidonoylglycerol in the LHb, while intra-LHb CB1R blockade increased basal corticosterone, augmented proactive coping strategies, and reduced anxiety-like behavior. Basal LHb 2-arachidonoylglycerol content was similarly elevated in rats that were subjected to chronic unpredictable stress or social defeat stress and positively correlated with adrenal weight. Finally, intra-LHb CB1R blockade increased proactive behaviors in response to a novel conspecific, increasing approach behaviors irrespective of stress history and decreasing the latency to be attacked during an agonistic encounter.
Conclusions: Alterations in LHb ECB signaling may be relevant for development of stress-related pathologies in which LHb dysfunction and stress-coping impairments are hallmark symptoms.
Keywords: Anxiety; CB(1) receptor; Endocannabinoid; Lateral habenula; Rat; Stress coping.
Published by Elsevier Inc.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures


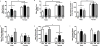


Comment in
-
Endocannabinoid Signaling in the Habenula Regulates Adaptive Responses to Stress.Biol Psychiatry. 2018 Oct 15;84(8):553-554. doi: 10.1016/j.biopsych.2018.08.001. Biol Psychiatry. 2018. PMID: 30261976 No abstract available.
References
-
- Hermans EJ, Henckens MJ, Joëls M, Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 1990;37:304–314. - PubMed
-
- Koolhaas JM, De Boer SF, Buwalda B, Van Reenen K. Individual variation in coping with stress: A multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol. 2007;70:218–226. - PubMed
-
- De Boer S, Buwalda B, Koolhaas J. Untangling the neurobiology of coping styles in rodents: towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci Biobehav Rev. 2016;16:30222–30226. - PubMed
-
- Veenema AH, Koolhaas JM, De Kloet ER. Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann NY Acad Sci. 2004;1018:255–265. - PubMed
-
- Veenema AH, Cremers TI, Jongsma ME, Steenbergen PJ, De Boer SF, Koolhaas JM. Differences in the effects of 5-HT1A receptor agonists on forced swimming behavior and brain 5-HT metabolism between low and high aggressive mice. Psychopharmacology (Berl) 2005;178:151–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

