Paneth Cell Multipotency Induced by Notch Activation following Injury
- PMID: 29887318
- PMCID: PMC6035085
- DOI: 10.1016/j.stem.2018.05.002
Paneth Cell Multipotency Induced by Notch Activation following Injury
Abstract
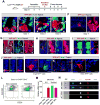
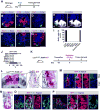
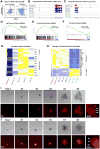
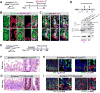
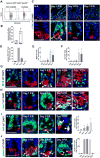
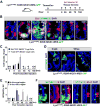
Paneth cells are post-mitotic intestinal epithelial cells supporting the stem cell niche and mucosal immunity. Paneth cell pathologies are observed in various gastrointestinal diseases, but their plasticity and response to genomic and environmental challenges remain unclear. Using a knockin allele engineered at the mouse Lyz1 locus, we performed detailed Paneth cell-lineage tracing. Irradiation induced a subset of Paneth cells to proliferate and differentiate into villus epithelial cells. RNA sequencing (RNA-seq) revealed that Paneth cells sorted from irradiated mice acquired a stem cell-like transcriptome; when cultured in vitro, these individual Paneth cells formed organoids. Irradiation activated Notch signaling, and forced expression of Notch intracellular domain (NICD) in Paneth cells, but not Wnt/β-catenin pathway activation, induced their dedifferentiation. This study documents Paneth cell plasticity, particularly their ability to participate in epithelial replenishment following stem cell loss, adding to a growing body of knowledge detailing the molecular pathways controlling injury-induced regeneration.
Keywords: Lyz1; Notch; Paneth cell; RNA-seq; Wnt; intestinal stem cell; lysozyme; plasticity.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. - PubMed
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

