A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain
- PMID: 29887379
- PMCID: PMC6559361
- DOI: 10.1016/j.cell.2018.05.022
A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain
Abstract
Noncoding RNAs (ncRNAs) play increasingly appreciated gene-regulatory roles. Here, we describe a regulatory network centered on four ncRNAs-a long ncRNA, a circular RNA, and two microRNAs-using gene editing in mice to probe the molecular consequences of disrupting key components of this network. The long ncRNA Cyrano uses an extensively paired site to miR-7 to trigger destruction of this microRNA. Cyrano-directed miR-7 degradation is much more effective than previously described examples of target-directed microRNA degradation, which come primarily from studies of artificial and viral RNAs. By reducing miR-7 levels, Cyrano prevents repression of miR-7-targeted mRNAs and enables accumulation of Cdr1as, a circular RNA known to regulate neuronal activity. Without Cyrano, excess miR-7 causes cytoplasmic destruction of Cdr1as in neurons, in part through enhanced slicing of Cdr1as by a second miRNA, miR-671. Thus, several types of ncRNAs can collaborate to establish a sophisticated regulatory network.
Keywords: TDMD; circRNA; lncRNA knockout; mammalian gene regulation; miRNA knockout; miRNA tailing; miRNA target-site disruption; miRNA trimming; noncoding RNA; regulatory network.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
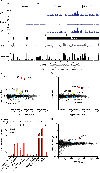

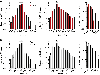



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

