A Flat BAR Protein Promotes Actin Polymerization at the Base of Clathrin-Coated Pits
- PMID: 29887380
- PMCID: PMC6057269
- DOI: 10.1016/j.cell.2018.05.020
A Flat BAR Protein Promotes Actin Polymerization at the Base of Clathrin-Coated Pits
Abstract
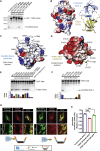
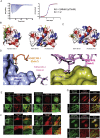
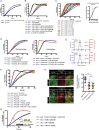
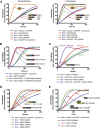
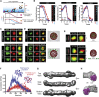
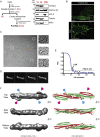
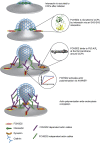
Multiple proteins act co-operatively in mammalian clathrin-mediated endocytosis (CME) to generate endocytic vesicles from the plasma membrane. The principles controlling the activation and organization of the actin cytoskeleton during mammalian CME are, however, not fully understood. Here, we show that the protein FCHSD2 is a major activator of actin polymerization during CME. FCHSD2 deletion leads to decreased ligand uptake caused by slowed pit maturation. FCHSD2 is recruited to endocytic pits by the scaffold protein intersectin via an unusual SH3-SH3 interaction. Here, its flat F-BAR domain binds to the planar region of the plasma membrane surrounding the developing pit forming an annulus. When bound to the membrane, FCHSD2 activates actin polymerization by a mechanism that combines oligomerization and recruitment of N-WASP to PI(4,5)P2, thus promoting pit maturation. Our data therefore describe a molecular mechanism for linking spatiotemporally the plasma membrane to a force-generating actin platform guiding endocytic vesicle maturation.
Keywords: ARP2/3; BAR domain; FCHSD2; N-WASP activation; Nervous Wreck; actin cytoskeleton; clathrin-mediated endocytosis; cytoskeletal forces; intersectin; membrane deformation.
Copyright © 2018 MRC Laboratory of Molecular Biology. Published by Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

