Biosynthesis of the nickel-pincer nucleotide cofactor of lactate racemase requires a CTP-dependent cyclometallase
- PMID: 29887527
- PMCID: PMC6093250
- DOI: 10.1074/jbc.RA118.003741
Biosynthesis of the nickel-pincer nucleotide cofactor of lactate racemase requires a CTP-dependent cyclometallase
Abstract
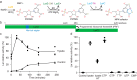
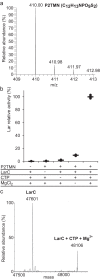
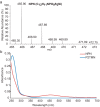
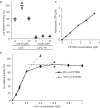
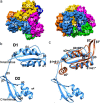
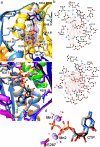
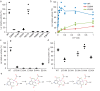
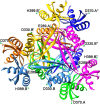
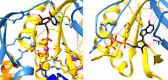
Bacterial lactate racemase is a nickel-dependent enzyme that contains a cofactor, nickel pyridinium-3,5-bisthiocarboxylic acid mononucleotide, hereafter named nickel-pincer nucleotide (NPN). The LarC enzyme from the bacterium Lactobacillus plantarum participates in NPN biosynthesis by inserting nickel ion into pyridinium-3,5-bisthiocarboxylic acid mononucleotide. This reaction, known in organometallic chemistry as a cyclometalation, is characterized by the formation of new metal-carbon and metal-sulfur σ bonds. LarC is therefore the first cyclometallase identified in nature, but the molecular mechanism of LarC-catalyzed cyclometalation is unknown. Here, we show that LarC activity requires Mn2+-dependent CTP hydrolysis. The crystal structure of the C-terminal domain of LarC at 1.85 Å resolution revealed a hexameric ferredoxin-like fold and an unprecedented CTP-binding pocket. The loss-of-function of LarC variants with alanine variants of acidic residues leads us to propose a carboxylate-assisted mechanism for nickel insertion. This work also demonstrates the in vitro synthesis and purification of the NPN cofactor, opening new opportunities for the study of this intriguing cofactor and of NPN-utilizing enzymes.
Keywords: bacterial metabolism; cyclometalation; lactate racemase; lactic acid; natural product biosynthesis; nickel; pincer complex; protein structure.
© 2018 Desguin et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

