Thrombus Formation and Propagation in the Onset of Cardiovascular Events
- PMID: 29887539
- PMCID: PMC6099067
- DOI: 10.5551/jat.RV17022
Thrombus Formation and Propagation in the Onset of Cardiovascular Events
Abstract
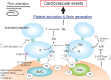
Ischemic cardiovascular disease is a major cause of morbidity and mortality worldwide and thrombus formation on disrupted atherosclerotic plaques is considered to trigger its onset. Although the activation of platelets and coagulation pathways has been investigated intensively, the mechanisms of thrombus formation on disrupted plaques have not been understood in detail. Platelets are thought to play a central role in the formation of arterial thrombus because of rapid flow conditions; however, thrombus that develops on disrupted plaques consistently includes large amounts of fibrin in addition to aggregated platelets. While, thrombus does not always become large enough to completely occlude the vascular lumen, indicating that the propagation of thrombus is also critical for the onset of cardiovascular events. Various factors, such as vascular wall thrombogenicity, altered blood flow and imbalanced blood hemostasis, modulate thrombus formation and propagation on disrupted plaques. Pathological findings derived from humans and experimental animal models of atherothrombosis have identified important factors that affect thrombus formation and propagation, namely platelets, extrinsic and intrinsic coagulation factors, proinflammatory factors, plaque hypoxia and blood flow alteration. These findings might provide insight into the mechanisms of thrombus formation and propagation on disrupted plaques that lead to the onset of cardiovascular events.
Keywords: Atherothrombosis; Blood flow; Factor Ⅺ; Platelet; Tissue factor.
Conflict of interest statement
None declared
Figures


References
-
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995; 92: 657-671 - PubMed
-
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000; 20: 1262-1275 - PubMed
-
- Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003; 41: 15S-22S - PubMed
-
- Komohara Y, Fujiwara Y, Ohnishi K, Shiraishi D, Takeya M. Contribution of macrophage polarization to metabolic diseases. J Atheroscler Thromb. 2016; 23: 10-17 - PubMed
-
- Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013; 368: 2004-2013 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

