The causal relationship between epigenetic abnormality and cancer development: in vivo reprogramming and its future application
- PMID: 29887568
- PMCID: PMC6085517
- DOI: 10.2183/pjab.94.016
The causal relationship between epigenetic abnormality and cancer development: in vivo reprogramming and its future application
Abstract
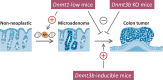
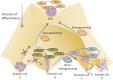
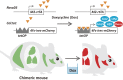
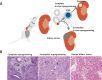
There is increasing evidence that cancer cells acquire epigenetic abnormalities as well as genetic mutations during cancer initiation, maintenance, and progression. However, the role of epigenetic regulation in cancer development, especially at the organismal level, remains to be elucidated. Here, we describe the causative role of epigenetic abnormalities in cancer, referring to our in vivo studies using induced pluripotent stem cell technology. We first summarize epigenetic reorganization during cellular reprogramming and introduce our in vivo reprogramming system for investigating the impact of dedifferentiation-driven epigenetic disruption in cancer development. Accordingly, we propose that particular types of cancer, in which causative mutations are not often detectable, such as pediatric cancers like Wilms' tumor, may develop mainly through alterations in epigenetic regulation triggered by dedifferentiation. Finally, we discuss issues that still remain to be resolved, and propose possible future applications of in vivo reprogramming to study cancer and other biological phenomena including organismal aging.
Keywords: cancer; epigenetics; iPS cell; in vivo reprogramming.
Figures
Similar articles
-
Concise review: dedifferentiation meets cancer development: proof of concept for epigenetic cancer.Stem Cells Transl Med. 2014 Oct;3(10):1182-7. doi: 10.5966/sctm.2014-0090. Epub 2014 Aug 13. Stem Cells Transl Med. 2014. PMID: 25122691 Free PMC article. Review.
-
Induced pluripotent stem cell technology for dissecting the cancer epigenome.Cancer Sci. 2015 Oct;106(10):1251-6. doi: 10.1111/cas.12758. Epub 2015 Sep 25. Cancer Sci. 2015. PMID: 26224327 Free PMC article. Review.
-
Cellular reprogramming technology for dissecting cancer epigenome in vivo.Epigenomics. 2017 Jul;9(7):997-1011. doi: 10.2217/epi-2017-0018. Epub 2017 Jun 27. Epigenomics. 2017. PMID: 28651445 Review.
-
Cellular reprogramming and cancer development.Int J Cancer. 2013 Mar 15;132(6):1240-8. doi: 10.1002/ijc.27963. Epub 2012 Dec 17. Int J Cancer. 2013. PMID: 23180619 Review.
-
Unveiling epigenetic regulation in cancer, aging, and rejuvenation with in vivo reprogramming technology.Cancer Sci. 2018 Sep;109(9):2641-2650. doi: 10.1111/cas.13731. Epub 2018 Aug 15. Cancer Sci. 2018. PMID: 29989289 Free PMC article. Review.
Cited by
-
Dedifferentiation and in vivo reprogramming of committed cells in wound repair (Review).Mol Med Rep. 2022 Dec;26(6):369. doi: 10.3892/mmr.2022.12886. Epub 2022 Nov 2. Mol Med Rep. 2022. PMID: 36321786 Free PMC article. Review.
-
LncRNA MORT (ZNF667-AS1) in Cancer-Is There a Possible Role in Gynecological Malignancies?Int J Mol Sci. 2021 Jul 22;22(15):7829. doi: 10.3390/ijms22157829. Int J Mol Sci. 2021. PMID: 34360598 Free PMC article. Review.
-
Non-invasive colorectal cancer biomarkers: HAND2 and GPM6A methylation in circulating tumour DNA.Cancer Cell Int. 2025 Jul 18;25(1):269. doi: 10.1186/s12935-025-03898-5. Cancer Cell Int. 2025. PMID: 40682031 Free PMC article.
-
In Vivo Reprogramming Highlights Epigenetic Regulation That Shapes Cancer Hallmarks.Cancer Sci. 2025 Jul;116(7):1807-1814. doi: 10.1111/cas.70067. Epub 2025 Apr 21. Cancer Sci. 2025. PMID: 40259515 Free PMC article. Review.
-
Epidrugs: targeting epigenetic marks in cancer treatment.Epigenetics. 2019 Dec;14(12):1164-1176. doi: 10.1080/15592294.2019.1640546. Epub 2019 Jul 13. Epigenetics. 2019. PMID: 31282279 Free PMC article. Review.
References
-
- Fearon E.R., Vogelstein B. (1990) A genetic model for colorectal tumorigenesis. Cell 61, 759–767. - PubMed
-
- Moser A.R., Pitot H.C., Dove W.F. (1990) A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324. - PubMed
-
- Wu C., Morris J.R. (2001) Genes, genetics, and epigenetics: a correspondence. Science 293, 1103–1105. - PubMed
-
- Feinberg A.P., Vogelstein B. (1983) Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 301, 89–92. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

