Synergistic effect of a LPEMF and SPIONs on BMMSC proliferation, directional migration, and osteoblastogenesis
- PMID: 29887957
- PMCID: PMC5992538
Synergistic effect of a LPEMF and SPIONs on BMMSC proliferation, directional migration, and osteoblastogenesis
Abstract
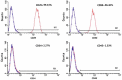
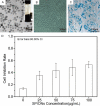
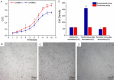
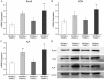
Pulsed electromagnetic fields (PEMFs) represent a new type of physiotherapy that has been shown to be effective for improving bone fracture healing and treating osteoporosis. Targeted therapy with bone marrow mesenchymal stem cells (BMMSCs) has been the focus of several recent studies. The key to such therapy is the effective application of certain nanomaterials in BMMSCs so they achieve an ideal target concentration under the influence of a PEMF. In our present study, the effects of a PEMF on the process of osteoblastogenesis were systematically investigated using superparamagnetic iron oxide nanoparticle (SPION)-labeled BMMSCs. Rat BMMSCs labeled with SPIONs were exposed to a low-frequency pulsed electromagnetic field (LPEMF) of 50 Hz at 1.1 mT. Exposure to the LPEMF resulted in an enhanced proliferation of SPION-labeled BMMSCs when compared with a control group. Furthermore, observations made by transmission electron microscopy (TEM) revealed greater cell concentrations in the central zone with exposure to the LPEMF than in the peripheral zone without LPEMF stimulation, indicating that a LPEMF could induce the migration of SPION-labeled BMMSCs towards a magnetic field. Transwell experiments confirmed that combining SPIONs with a LPEMF could significantly promote the directional migration of BMMSCs. Von Kossa and ALP staining of LPEMF-exposed SPION-labeled cells was more intense, and those cells displayed higher levels of ALP activity than control cells. The SPION-labeled, LPEMF-exposed cells also showed increased levels of osteogenesis-related gene and protein expression (e.g., ALP, OCN, and RUNX2) in PCR and western blot studies. Taken together, our findings suggest that a combination of LPEMF and SPIONs exerts a synergistic effect on promoting the directional migration and osteogenic differentiation of BMMSCs, indicating that application of a LPEMF in conjunction with SPIONs may constitute a method for treating bone defects.
Keywords: Pulsed electromagnetic fields; bone marrow mesenchymal stem cells; osteoblastogenesis; superparamagnetic iron oxide nanoparticles.
Conflict of interest statement
None.
Figures

Similar articles
-
Low frequency‑pulsed electromagnetic fields promote osteogenic differentiation of bone marrow‑derived mesenchymal stem cells by regulating connexin 43 expression.Exp Ther Med. 2024 Oct 1;28(6):446. doi: 10.3892/etm.2024.12736. eCollection 2024 Dec. Exp Ther Med. 2024. PMID: 39386938 Free PMC article.
-
Pulsed electromagnetic field induces Ca2+-dependent osteoblastogenesis in C3H10T1/2 mesenchymal cells through the Wnt-Ca2+/Wnt-β-catenin signaling pathway.Biochem Biophys Res Commun. 2018 Sep 5;503(2):715-721. doi: 10.1016/j.bbrc.2018.06.066. Epub 2018 Jun 18. Biochem Biophys Res Commun. 2018. PMID: 29909008
-
Enhanced effect of combining bone marrow mesenchymal stem cells (BMMSCs) and pulsed electromagnetic fields (PEMF) to promote recovery after spinal cord injury in mice.MedComm (2020). 2022 Aug 3;3(3):e160. doi: 10.1002/mco2.160. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35949547 Free PMC article.
-
Effect of pulsed electromagnetic field on the proliferation and differentiation potential of human bone marrow mesenchymal stem cells.Bioelectromagnetics. 2009 May;30(4):251-60. doi: 10.1002/bem.20472. Bioelectromagnetics. 2009. PMID: 19204973
-
Biological Scaffolds Assembled with Magnetic Nanoparticles for Bone Tissue Engineering: A Review.Materials (Basel). 2023 Feb 8;16(4):1429. doi: 10.3390/ma16041429. Materials (Basel). 2023. PMID: 36837058 Free PMC article. Review.
Cited by
-
Exposure of primary osteoblasts to combined magnetic and electric fields induced spatiotemporal endochondral ossification characteristic gene- and protein expression profiles.J Exp Orthop. 2022 May 2;9(1):39. doi: 10.1186/s40634-022-00477-9. J Exp Orthop. 2022. PMID: 35499653 Free PMC article.
-
Biophysical control of plasticity and patterning in regeneration and cancer.Cell Mol Life Sci. 2023 Dec 15;81(1):9. doi: 10.1007/s00018-023-05054-6. Cell Mol Life Sci. 2023. PMID: 38099951 Free PMC article. Review.
-
The Role of Low-Frequency Electromagnetic Fields on Mesenchymal Stem Cells Differentiation: A Systematic Review.Tissue Eng Regen Med. 2022 Dec;19(6):1147-1160. doi: 10.1007/s13770-022-00473-1. Epub 2022 Aug 30. Tissue Eng Regen Med. 2022. PMID: 36042129 Free PMC article.
-
Pulsed Electromagnetic Field Therapy and Direct Current Electric Field Modulation Promote the Migration of Fibroblast-like Synoviocytes to Accelerate Cartilage Repair In Vitro.Appl Sci (Basel). 2022 Dec 1;12(23):12406. doi: 10.3390/app122312406. Epub 2022 Dec 4. Appl Sci (Basel). 2022. PMID: 36970107 Free PMC article.
-
Magnetic ion channel activation of TREK1 in human mesenchymal stem cells using nanoparticles promotes osteogenesis in surrounding cells.J Tissue Eng. 2018 Oct 30;9:2041731418808695. doi: 10.1177/2041731418808695. eCollection 2018 Jan-Dec. J Tissue Eng. 2018. PMID: 30397432 Free PMC article.
References
-
- Reid I. Anti-resorptive therapies for osteoporosis. Semin Cell Dev Biol. 2008;19:473–478. - PubMed
-
- Brommage R. Genetic approaches to identifying novel osteoporosis drug targets. J Cell Biochem. 2015;116:2139–2145. - PubMed
-
- Fulfaro F, Casuccio A, Ticozzi C, Ripamonti C. The role of bisphosphonates in the treatment of painful metastatic bone disease: a review of phase III trials. Pain. 1998;78:157–169. - PubMed
-
- Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: physiological complexity belies therapeutic simplicity. Science. 2004;304:1269–1273. - PubMed
-
- Li X, Zhang M, Bai L, Bai W, Xu W, Zhu H. Effects of 50 Hz pulsed electromagnetic fields on the growth and cell cycle arrest of mesenchymal stem cells: an in vitro study. Electromagn Biol Med. 2012;31:356–364. - PubMed
LinkOut - more resources
Full Text Sources
