Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing
- PMID: 29887978
- PMCID: PMC5990937
- DOI: 10.1080/20013078.2018.1481321
Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing
Erratum in
-
Erratum: Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by Next-Generation Sequencing.J Extracell Vesicles. 2019 Mar 8;8(1):1581487. doi: 10.1080/20013078.2019.1581487. eCollection 2019. J Extracell Vesicles. 2019. PMID: 30891163 Free PMC article.
Abstract
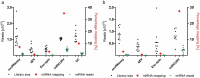
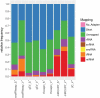
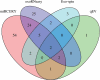
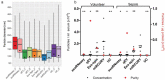
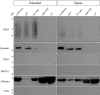
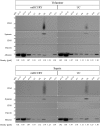
Extracellular vesicles (EVs) are intercellular communicators with key functions in physiological and pathological processes and have recently garnered interest because of their diagnostic and therapeutic potential. The past decade has brought about the development and commercialization of a wide array of methods to isolate EVs from serum. Which subpopulations of EVs are captured strongly depends on the isolation method, which in turn determines how suitable resulting samples are for various downstream applications. To help clinicians and scientists choose the most appropriate approach for their experiments, isolation methods need to be comparatively characterized. Few attempts have been made to comprehensively analyse vesicular microRNAs (miRNAs) in patient biofluids for biomarker studies. To address this discrepancy, we set out to benchmark the performance of several isolation principles for serum EVs in healthy individuals and critically ill patients. Here, we compared five different methods of EV isolation in combination with two RNA extraction methods regarding their suitability for biomarker discovery-focused miRNA sequencing as well as biological characteristics of captured vesicles. Our findings reveal striking method-specific differences in both the properties of isolated vesicles and the ability of associated miRNAs to serve in biomarker research. While isolation by precipitation and membrane affinity was highly suitable for miRNA-based biomarker discovery, methods based on size-exclusion chromatography failed to separate patients from healthy volunteers. Isolated vesicles differed in size, quantity, purity and composition, indicating that each method captured distinctive populations of EVs as well as additional contaminants. Even though the focus of this work was on transcriptomic profiling of EV-miRNAs, our insights also apply to additional areas of research. We provide guidance for navigating the multitude of EV isolation methods available today and help researchers and clinicians make an informed choice about which strategy to use for experiments involving critically ill patients.
Keywords: Extracellular vesicle; biomarker; exosome isolation; miRNA; next-generation sequencing; precipitation; sepsis; small RNA sequencing; ultracentrifugation.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

