Biomaterials at the interface of nano- and micro-scale vector-cellular interactions in genetic vaccine design
- PMID: 29887986
- PMCID: PMC5990286
- DOI: 10.1039/C4TB01058B
Biomaterials at the interface of nano- and micro-scale vector-cellular interactions in genetic vaccine design
Abstract
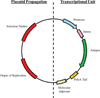
The development of safe and effective vaccines for the prevention of elusive infectious diseases remains a public health priority. Immunization, characterized by adaptive immune responses to specific antigens, can be raised by an array of delivery vectors. However, current commercial vaccination strategies are predicated on the retooling of archaic technology. This review will discuss current and emerging strategies designed to elicit immune responses in the context of genetic vaccination. Selected strategies at the biomaterial-biological interface will be emphasized to illustrate the potential of coupling both fields towards a common goal.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

