Comparison between Different Extraction Methods for Determination of Primary Aromatic Amines in Food Simulant
- PMID: 29888024
- PMCID: PMC5977034
- DOI: 10.1155/2018/1651629
Comparison between Different Extraction Methods for Determination of Primary Aromatic Amines in Food Simulant
Erratum in
-
Erratum to "Comparison between Different Extraction Methods for Determination of Primary Aromatic Amines in Food Simulant".J Anal Methods Chem. 2018 Sep 6;2018:6423428. doi: 10.1155/2018/6423428. eCollection 2018. J Anal Methods Chem. 2018. PMID: 30271653 Free PMC article.
Abstract
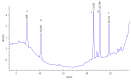
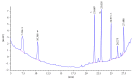
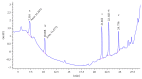
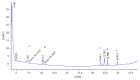
The primary aromatic amines (PAAs) are food contaminants which may exist in packaged food. Polyurethane (PU) adhesives which are used in flexible packaging are the main source of PAAs. It is the unreacted diisocyanates which in fact migrate to foodstuff and then hydrolyze to PAAs. These PAAs include toluenediamines (TDAs) and methylenedianilines (MDAs), and the selected PAAs were 2,4-TDA, 2,6-TDA, 4,4'-MDA, 2,4'-MDA, and 2,2'-MDA. PAAs have genotoxic, carcinogenic, and allergenic effects. In this study, extraction methods were applied on a 3% acetic acid as food simulant which was spiked with the PAAs under study. Extraction methods were liquid-liquid extraction (LLE), dispersive liquid-liquid microextraction (DLLME), and solid-phase extraction (SPE) with C18 ec (octadecyl), HR-P (styrene/divinylbenzene), and SCX (strong cationic exchange) cartridges. Extracted samples were detected and analyzed by HPLC-UV. In comparison between methods, recovery rate of SCX cartridge showed the best adsorption, up to 91% for polar PAAs (TDAs and MDAs). The interested PAAs are polar and relatively soluble in water, so a cartridge with cationic exchange properties has the best absorption and consequently the best recoveries.
Figures


References
-
- Sennbro C. J., Lindh C. H., Tinnerberg H., et al. Development, validation and characterization of an analytical method for the quantification of hydrolysable urinary metabolites and plasma protein adducts of 2,4- and 2,6-toluene diisocyanate, 1,5-naphthalene diisocyanate and 4,4’-methylenediphenyl diisocyanate. 2003;8(3-4):204–217. doi: 10.1080/1354750031000090660. - DOI - PubMed
-
- Oostdyk T. S., Grob R. L., Snyder J. L., McNally M. E. Solid-phase extraction of primary aromatic amines from aqueous samples; comparison with liquid-liquid extraction techniques. 1994;29(8):1607–1628. doi: 10.1080/10934529409376135. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

