Circulating Neurotoxic 5-HT2A Receptor Agonist Autoantibodies in Adult Type 2 Diabetes with Parkinson's Disease
- PMID: 29888323
- PMCID: PMC5990037
- DOI: 10.15226/2374-6890/5/2/01102
Circulating Neurotoxic 5-HT2A Receptor Agonist Autoantibodies in Adult Type 2 Diabetes with Parkinson's Disease
Abstract
Aims: To test whether circulating neurotoxic autoantibodies increase in adult type 2 diabetes mellitus with Parkinson's disease (PD) or dementia. To identify the G-protein coupled receptor on neuroblastoma cells mediating neural inhibitory effects in diabetic Parkinson's disease plasma autoantibodies. To determine the mechanism of accelerated neuroblastoma cell death and acute neurite retraction induced by diabetic Parkinson's disease and dementia autoantibodies.
Methods: Protein-A eluates from plasma of twelve older adult male diabetic patients having Parkinson's disease (n=10) or dementia (n=2), and eight age-matched control diabetic patients were tested for ability to cause accelerated N2A neuroblastoma cell death and acute neurite retraction. Specific antagonists of G protein coupled receptors belonging to the G alpha q subfamily of heterotrimetric G-proteins, the phospholipase C/inositol triphosphate/Ca2+ pathway, or the RhoA/Rho kinase pathway were tested for ability to block diabetic Parkinson's disease/dementia autoantibody-induced neurite retraction or N2A accelerated cell loss. Sequential Liposorber LA-15 dextran sulfate cellulose/protein-A affinity chromatography was used to obtain highly-purified fractions of diabetic Parkinson's disease autoantibodies.
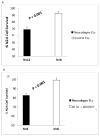
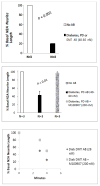
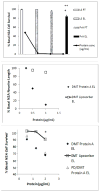
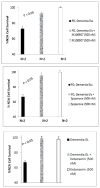
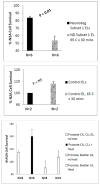
Results: Mean accelerated neuroblastoma cell loss induced by diabetic Parkinson's disease or dementia autoantibodies significantly exceeded (P = 0.001) the level of N2A cell loss induced by an identical concentration of the diabetic autoantibodies in control patients without these two co-morbid neurodegenerative disorders. Co-incubation of diabetic Parkinson's disease and dementia autoantibodies with two-hundred nanomolar concentrations of M100907, a highly selective 5-HT2AR antagonist, completely prevented autoantibody-induced accelerated N2A cell loss and neurite retraction. A higher concentration (500 nM-10μM) of alpha-1 adrenergic, angiotensin II type 1, or endothelin A receptor antagonists did not substantially inhibit autoantibody-induced neuroblastoma cell death or prevent neurite retraction. Antagonists of the inositol triphosphate receptor (2-APB, 50μM), the intracellular calcium chelator (BAPTA-AM, 30 μM) and Y27632 (10 μM), a selective RhoA/Rho kinase inhibitor, each completely blocked acute neurite retraction induced by sixty nanomolar concentrations of diabetic Parkinson's disease autoantibodies. Co-incubation with 2-APB (1-2 μM) for 8 hours' prevented autoantibody-induced N2A cell loss. The highly-purified fraction obtained after Liposorber LA/protein-A affinity chromatography in hypertriglyceridemic diabetic dementia and Parkinson's disease plasmas had apparent MWs > 30 kD, and displayed enhanced N2A toxicity requiring substantially higher concentrations of 5-HT2AR antagonists (M100907, ketanserin, spiperone) to effectively neutralize.
Conclusion: These data suggest increased autoantibodies in older adult diabetes with Parkinson's disease or dementia cause accelerated neuron loss via the 5-hydroxytryptamine 2 receptor coupled to inositol triphosphate receptor-mediated cytosolic Ca2+ release.
Keywords: 5-HT2A receptor; Parkinson’s disease; autoantibodies; dementia; diabetes.
Conflict of interest statement
Conflict of Interest The author reports no financial conflict of interest that would affect the objectivity of the presented findings.
Figures


References
-
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–1022. - PubMed
-
- Xu T, Pandey SC. Cellular localization of serotonin (2A) (5HT (2A)) receptors in the rat brain. Brain Res Bull. 2000;51(6):499–505. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
