Electrocorticography reveals continuous auditory and visual speech tracking in temporal and occipital cortex
- PMID: 29888819
- PMCID: PMC6289876
- DOI: 10.1111/ejn.13992
Electrocorticography reveals continuous auditory and visual speech tracking in temporal and occipital cortex
Abstract
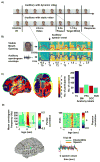
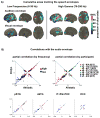
During natural speech perception, humans must parse temporally continuous auditory and visual speech signals into sequences of words. However, most studies of speech perception present only single words or syllables. We used electrocorticography (subdural electrodes implanted on the brains of epileptic patients) to investigate the neural mechanisms for processing continuous audiovisual speech signals consisting of individual sentences. Using partial correlation analysis, we found that posterior superior temporal gyrus (pSTG) and medial occipital cortex tracked both the auditory and the visual speech envelopes. These same regions, as well as inferior temporal cortex, responded more strongly to a dynamic video of a talking face compared to auditory speech paired with a static face. Occipital cortex and pSTG carry temporal information about both auditory and visual speech dynamics. Visual speech tracking in pSTG may be a mechanism for enhancing perception of degraded auditory speech.
Keywords: audiovisual speech; clear speech; continuous speech; multisensory; naturalistic stimuli.
© 2018 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest to declare.
Figures

References
-
- Arnal LH, Wyart V, Giraud AL. Transitions in neural oscillations reflect prediction errors generated in audiovisual speech. Nat Neurosci. 2011;14:797–801. - PubMed
-
- Ashburner J, Friston KJ. Computing average shaped tissue probability templates. NeuroImage. 2009;45(2):333–341. - PubMed
-
- Beauchamp MS, Lee KE, Argall BD, Martin A. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 2004;41:809–823. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

