Promoter methylation of PGC1A and PGC1B predicts cancer incidence in a veteran cohort
- PMID: 29888964
- PMCID: PMC6367713
- DOI: 10.2217/epi-2017-0141
Promoter methylation of PGC1A and PGC1B predicts cancer incidence in a veteran cohort
Abstract
Aim: Previous studies suggest telomere shortening represses PGC1A and PGC1B expression leading to mitochondrial dysfunction. Methylation of CpG sites within these genes may interact with these factors to affect cancer risk.
Materials & methods: Among 385 men, we identified 84 incidents of cancers (predominantly prostate and nonmelanoma skin). We examined associations between leukocyte DNA methylation of 41 CpGs from PGC1A and PGC1B with telomere length, mitochondrial 8-OHdG lesions, mitochondrial abundance and cancer incidence.
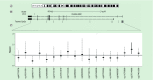
Results: Methylation of five and eight CpG sites were significantly associated with telomere length and mitochondrial abundance at p < 0.05. Two CpG sites were independently associated with cancer risk: cg27514608 (PGC1A, TSS1500; HR: 1.55, 95% CI: 1.19-2.03, FDR = 0.02), and cg15219393 (PGC1B, first exon/5'UTR; HR: 3.71, 95% CI: 1.82-7.58, FDR < 0.01). Associations with cg15219393 were observed primarily among men with shorter leukocyte telomeres.
Conclusion: PGC1A and PGC1B methylation may serve as early biomarkers of cancer risk.
Keywords: DNA methylation; cancer biomarkers; cancer epigenetics.
Conflict of interest statement
The Epidemiology Research and Information Center of US Department of Veterans Affairs (NIEHS R01-ES015172) support the Normative Aging Study, which is a research component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC). L Hou, C Achenbach and R Murphy received support from the National Cancer Institute (U54-CA221205). L Hou received additional support from the Northwestern University Robert H Lurie Comprehensive Cancer Center Rosenberg Research Fund. A Baccarelli and J Schwartz received additional support from the National Institute of Environmental Health Sciences (NIEHS R01-ES021733 and NIEHS P30-ES00002). J Kresovich received additional support from the National Cancer Institute Cancer Education and Career Development Program (NIH R25-CA057699). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures

References
-
- Navarrete-Reyes AP, Soto-Pérez-de-Celis E, Hurria A. Cancer and aging: a complex biological association. Rev. Invest. Clin. 2016;68(1):17–24. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67(1):7–30. - PubMed
-
- Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448(7155):767–774. - PubMed
-
- Kong Y, Trabucco SE, Zhang H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Interdiscip. Top. Gerontol. 2014;39:86–107. - PubMed
-
- Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp. Biol. Med. (Maywood) 2013;238(5):450–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
