Oesophageal stenting: Status quo and future challenges
- PMID: 29888981
- PMCID: PMC6475941
- DOI: 10.1259/bjr.20170935
Oesophageal stenting: Status quo and future challenges
Abstract
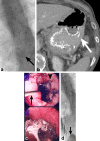
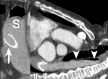
Oesophageal stents are widely used for palliating dysphagia from malignant obstruction. They are also used with increasing frequency in the treatment of oesophageal perforation, as well as benign strictures from a variety of causes. Improved oncological treatments have led to prolonged survival of patients treated with palliative intent; as a consequence, stents need to function and last longer in order to avoid repeat procedures. There is also increasing need for meticulous procedure planning, careful selection of the device most appropriate for the individual patient and planned follow-up. Furthermore, as more patients are cured, there will be more issues with resultant long-term side-effects, such as recalcitrant strictures due to radiotherapy or anastomotic scarring, which will have to be addressed. Stent design needs to keep up with the progress of cancer treatment, in order to offer patients the best possible long-term result. This review article attempts to illustrate the changing realities in oesophageal stenting, differences in current stent designs and behaviour, as well as the pressing need to refine and modify devices in order to meet the new challenges.
Figures







References
-
- Laasch H-U, Lee S, Moss J, Roobottom C, Kinsman R, Walton P. British Society of Interventional Radiology. ROST - Registry of Oesophageal Stenting, First Report 2004. Henley-on-Thames: The British Institute of Radiology.; 2004.
-
- Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. COUGAR-02 Investigators Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014; 15: 78–86. doi: 10.1016/S1470-2045(13)70549-7 - DOI - PubMed
-
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. European Society for Medical Oncology (ESMO) European Society of Surgical Oncology (ESSO) European Society of Radiotherapy and Oncology (ESTRO) Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl 6): vi57–vi63. doi: 10.1093/annonc/mdt344 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

