Dual antibiotics for bronchiectasis
- PMID: 29889304
- PMCID: PMC6513403
- DOI: 10.1002/14651858.CD012514.pub2
Dual antibiotics for bronchiectasis
Abstract
Background: Bronchiectasis is a chronic respiratory disease characterised by abnormal and irreversible dilatation of the smaller airways and associated with a mortality rate greater than twice that of the general population. Antibiotics serve as front-line therapy for managing bacterial load, but their use is weighed against the development of antibiotic resistance. Dual antibiotic therapy has the potential to suppress infection from multiple strains of bacteria, leading to more successful treatment of exacerbations, reduced symptoms, and improved quality of life. Further evidence is required on the efficacy of dual antibiotics in terms of management of exacerbations and extent of antibiotic resistance.
Objectives: To evaluate the effects of dual antibiotics in the treatment of adults and children with bronchiectasis.
Search methods: We identified studies from the Cochrane Airways Group Specialised Register (CAGR), which includes the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Allied and Complementary Medicine (AMED), and PsycINFO, as well as studies obtained by handsearching of journals/abstracts. We also searched the following trial registries: US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform. We imposed no restriction on language of publication. We conducted our search in October 2017.
Selection criteria: We searched for randomised controlled trials comparing dual antibiotics versus a single antibiotic for short-term (< 4 weeks) or long-term management of bronchiectasis diagnosed in adults and/or children by bronchography, plain film chest radiography, or high-resolution computed tomography. Primary outcomes included exacerbations, length of hospitalisation, and serious adverse events. Secondary outcomes were response rates, emergence of resistance to antibiotics, systemic markers of infection, sputum volume and purulence, measures of lung function, adverse events/effects, deaths, exercise capacity, and health-related quality of life. We did not apply outcome measures as selection criteria.
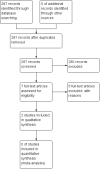
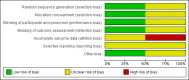
Data collection and analysis: Two review authors independently screened the titles and abstracts of 287 records, along with the full text of seven reports. Two studies met review inclusion criteria. Two review authors independently extracted outcome data and assessed risk of bias. We extracted data from only one study and conducted GRADE assessments for the following outcomes: successful treatment of exacerbation; response rates; and serious adverse events.
Main results: Two randomised trials assessed the effectiveness of oral plus inhaled dual therapy versus oral monotherapy in a total of 118 adults with a mean age of 62.8 years. One multi-centre trial compared inhaled tobramycin plus oral ciprofloxacin versus ciprofloxacin alone, and one single-centre trial compared nebulised gentamicin plus systemic antibiotics versus a systemic antibiotic alone. Published papers did not report study funding sources.Effect estimates from one small study with 53 adults showed no evidence of treatment benefit with oral plus inhaled dual therapy for the following primary outcomes at the end of the study: successful management of exacerbation - cure at day 42 (odds ratio (OR) 0.66, 95% confidence interval (CI) 0.22 to 2.01; 53 participants; one study; very low-quality evidence); number of participants with Pseudomonas aeruginosa eradication at day 21 (OR 2.33, 95% CI 0.66 to 8.24; 53 participants; one study; very low-quality evidence); and serious adverse events (OR 0.48, 95% CI 0.08 to 2.87; 53 participants; one study; very low-quality evidence). Similarly, researchers provided no evidence of treatment benefit for the following secondary outcomes: clinical response rates - relapse at day 42 (OR 0.57, 95% CI 0.12 to 2.69; 53 participants; one study; very low-quality evidence); microbiological response rate at day 21 - eradicated (OR 2.40, 95% CI 0.67 to 8.65; 53 participants; one study; very low-quality evidence); and adverse events - incidence of wheeze (OR 5.75, 95% CI 1.55 to 21.33). Data show no evidence of benefit in terms of sputum volume, lung function, or antibiotic resistance. Outcomes from a second small study with 65 adults, available only as an abstract, were not included in the quantitative data synthesis. The included studies did not report our other primary outcomes: duration; frequency; and time to next exacerbation; nor our secondary outcomes: systemic markers of infection; exercise capacity; and quality of life. We did not identify any trials that included children.
Authors' conclusions: A small number of studies in adults have generated high-quality evidence that is insufficient to inform robust conclusions, and studies in children have provided no evidence. We identified only one dual-therapy combination of oral and inhaled antibiotics. Results from this single trial of 53 adults that we were able to include in the quantitative synthesis showed no evidence of treatment benefit with oral plus inhaled dual therapy in terms of successful treatment of exacerbations, serious adverse events, sputum volume, lung function, and antibiotic resistance. Further high-quality research is required to determine the efficacy and safety of other combinations of dual antibiotics for both adults and children with bronchiectasis, particularly in terms of antibiotic resistance.
Conflict of interest statement
Sally Spencer, Dave Lynes, and Ross Armstrong are named co‐investigators on a study funded by Edge Hill University to develop a series of reviews on bronchiectasis; Lambert Felix is a part‐time review author supported by this funding; Haley Harrison received funding support through an NIHR internship. The remaining review authors did not receive funding for this systematic review.
Figures


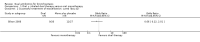
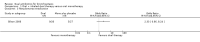









Update of
References
References to studies included in this review
Bilton 2006 {published data only}
-
- Bilton D, Henig N, Morrissey B, Gotfried M. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest 2006;130(5):1503‐10. [PUBMED: 17099030] - PubMed
Hossain 2010 {published data only}
-
- Hossain A, Ahamed M, Sharkar ZH. Change in clinical outcome of exacerbation of bronchiectasis on addition of nebulized gentamicin to systemic antibiotic. Respirology 2010;15(Suppl 2):38 (OS 08‐01).
References to studies excluded from this review
Orriols 1999 {published data only}
-
- Orriols R, Roig J, Ferrer J, Sampol G, Rosell A, Ferrer A, et al. Inhaled antibiotic therapy in non‐cystic fibrosis patients with bronchiectasis and chronic bronchial infection by Pseudomonas aeruginosa. Respiratory Medicine 1999;93(7):476‐80. [PUBMED: 10464834] - PubMed
Orriols 2015 {published data only}
-
- Orriols R, Hernando R, Ferrer A, Terradas S, Montoro B. Eradication therapy against Pseudomonas aeruginosa in non‐cystic fibrosis bronchiectasis. Respiration 2015;90(4):299‐305. [PUBMED: 26340658] - PubMed
Takamoto 1994 {published data only}
-
- Takamoto M, Ishibashi T, Toyoshima H, Tanaka H, Tamaru N, Watanabe K, et al. Imipenem/cilastatin sodium alone or combined with amikacin sulfate in respiratory infections. Japanese Journal of Antibiotics 1994;47(9):1131‐44. - PubMed
Vergnon 1985 {published data only}
-
- Vergnon JM, Vincent M, Ros A, Brun Y, Brune J. [Comparative clinical trial of cefoperazone versus ampicillin + tobramycin in severe bronchopulmonary and pleural infectious pathology] [Essai clinique comparatif cefoperazone contre ampicilline + tobramycine en pathologie infectieuse broncho‐pulmonaire et pleurale grave]. Revue de Pneumologie Clinique 1985;41(3):205‐11. [PUBMED: 4048752] - PubMed
Watanabe 1990 {published data only}
-
- Watanabe A, Motomiya M, Nukiwa T, Nakai Y, Honda Y, Konno K, et al. A well‐controlled comparative clinical study of the combination regimen of ciprofloxacin plus erythromycin for the treatment of repeated acute exacerbations of chronic respiratory tract infections. Chemotherapy 1994;42(10):1194‐201.
Additional references
Chalmers 2012
-
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short‐ and long‐term antibiotic treatment reduces airway and systemic inflammation in non–cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;186(7):657‐65. - PubMed
Chalmers 2014
Chang 2002
-
- Chang AB, Grimwood K, Mulholland EK, Torzillo PJ. Bronchiectasis in indigenous children in remote Australian communities. Medical Journal of Australia 2002;177(4):200‐4. [PUBMED: 12175325] - PubMed
Chang 2010
-
- Chang AB, Bell SC, Byrnes CA, Grimwood K, Holmes P, King PT, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. Medical Journal of Australia 2010;193(6):356‐65. - PubMed
Cole 1986
-
- Cole PJ. Inflammation: a two‐edged sword ‐ the model of bronchiectasis. European Journal of Respiratory Diseases Supplement 1986;147:6‐15. - PubMed
Cole 1997
-
- Cole P. The damaging role of bacteria in chronic lung infection. Journal of Antimicrobial Chemotherapy 1997;40(Suppl A):5‐10. - PubMed
Elphick 2005
European Lung White Book 2013
-
- Gibson GJ, Loddenkemper R, Sibille Y, Lundbäck B, editor(s). European Lung White Book: Respiratory Health and Disease in Europe. www.erswhitebook.org/ (accessed before 11 October 2017).
Evans 1996
-
- Evans SA, Turner SM, Bosch BJ, Hardy CC, Woodhead MA. Lung function in bronchiectasis: the influence of P seudomonas aeruginosa. European Respiratory Journal 1996;9(8):1601‐4. - PubMed
Evans 2003
-
- Evans DJ, Greenstone M. Long‐term antibiotics in the management of non‐CF bronchiectasis ‐ do they improve outcome?. Respiratory Medicine 2003;97(7):851‐8. - PubMed
Foweraker 2011
-
- Foweraker JWD. Microbiology of non‐CF bronchiectasis. European Respiratory Society Monograph 2011;52:68‐96.
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 15 July 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Habesoglu 2011
Hansen 2015
Haworth 2014
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kaehne 2017
Kapur 2012
-
- Kapur N, Grimwood K, Masters IB, Morris PS, Chang AB. Lower airway microbiology and cellularity in children with newly diagnosed non‐CF bronchiectasis. Pediatric Pulmonology 2012;47:300–7. - PubMed
Kapur 2012a
-
- Kapur N, Masters IB, Newcombe P, Chang AB. The burden of disease in pediatric non‐cystic fibrosis bronchiectasis. Chest 2012;141(4):1018‐24. [PUBMED: 21885727] - PubMed
Kelly 2018
Lavery 2005
-
- Lavery K, Bradley JM, Elborn JS. Bronchiectasis: challenges in diagnosis and management. International Journal of Respiratory Care 2005;1:92‐8.
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Martinez Garcia 2007
-
- Martinez‐Garcia MA, Soler‐Cataluna JJ, Perpina‐Tordera M, Roman‐Sanchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non‐cystic fibrosis bronchiectasis. Chest 2007;132(5):1565‐72. - PubMed
Martinez Garcia 2014
-
- Martinez‐Garcia MA, Gracia J, Relat MV, Giron RM, Carro LM, Rosa Carrillo D, et al. Multidimensional approach to non‐cystic fibrosis bronchiectasis: the FACED score. European Respiratory Journal 2014;43:1357‐67. - PubMed
McShane 2013
-
- McShane PJ, Naureckas ET, Tino G, Strek ME. Non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2013;188:647–56. - PubMed
Moher 2009
Pasteur 2010
-
- Pasteur MC, Bilton D, Hill AT. British Thoracic Society Bronchiectasis Non‐CF Guideline Group. British Thoracic Society guideline for non CF bronchiectasis. Thorax 2010;65:i1–i58. - PubMed
Polverino 2017
Quint 2016
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ringshausen 2015
-
- Ringshausen FC, Roux A, Diel R, Hohmann D, Welte T, Rademacher J. Bronchiectasis in Germany: a population‐based estimation of disease prevalence. European Respiratory Journal 2015; Vol. 46, issue 6:1805‐7. [PUBMED: 26293498] - PubMed
Roberts 2010
-
- Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respiratory Medicine 2010;104:981‐5. - PubMed
Rubin 2014
-
- Rubin BK, Williams RW. Aerosolized antibiotics for non‐cystic fibrosis bronchiectasis. Respiration 2014;88:177–84. - PubMed
Seitz 2010
Seitz 2012
Twiss 2005
Valery 2012
Weycker 2005
-
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12:205‐9.
Wilson 1997
-
- Wilson CB, Jones PW, O'Leary CJ, Hansell DM, Cole PJ, Wilson R. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. European Respiratory Journal 1997;10:1754‐60. - PubMed
Wu 2014
-
- Wu Q, Shen W, Cheng H, Zhou X. Long‐term macrolides for non‐cystic fibrosis bronchiectasis: a systematic review and meta‐analysis. Respirology 2014;19(3):321–9. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

