DNA adducts: Formation, biological effects, and new biospecimens for mass spectrometric measurements in humans
- PMID: 29889312
- PMCID: PMC6289887
- DOI: 10.1002/mas.21570
DNA adducts: Formation, biological effects, and new biospecimens for mass spectrometric measurements in humans
Abstract
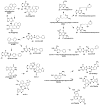
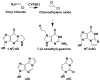
Hazardous chemicals in the environment and diet or their electrophilic metabolites can form adducts with genomic DNA, which can lead to mutations and the initiation of cancer. In addition, reactive intermediates can be generated in the body through oxidative stress and damage the genome. The identification and measurement of DNA adducts are required for understanding exposure and the causal role of a genotoxic chemical in cancer risk. Over the past three decades, 32 P-postlabeling, immunoassays, gas chromatography/mass spectrometry, and liquid chromatography/mass spectrometry (LC/MS) methods have been established to assess exposures to chemicals through measurements of DNA adducts. It is now possible to measure some DNA adducts in human biopsy samples, by LC/MS, with as little as several milligrams of tissue. In this review article, we highlight the formation and biological effects of DNA adducts, and highlight our advances in human biomonitoring by mass spectrometric analysis of formalin-fixed paraffin-embedded tissues, untapped biospecimens for carcinogen DNA adduct biomarker research.
Keywords: DNA adduct; biomarker; biomonitoring; carcinogen; mass spectrometry.
© 2018 Wiley Periodicals, Inc.
Figures


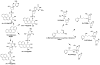

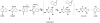









References
-
- Miller EC. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978;38:1479–1496. - PubMed
-
- Scharer OD. Chemistry and biology of DNA repair. Angew Chem Int Ed Engl. 2003;42:2946–2974. - PubMed
-
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. - PubMed
-
- Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

