Current Progress in Electrotransfection as a Nonviral Method for Gene Delivery
- PMID: 29889538
- PMCID: PMC6123289
- DOI: 10.1021/acs.molpharmaceut.8b00207
Current Progress in Electrotransfection as a Nonviral Method for Gene Delivery
Abstract
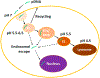
Electrotransfection (ET) is a nonviral method for delivery of various types of molecules into cells both in vitro and in vivo. Close to 90 clinical trials that involve the use of ET have been performed, and approximately half of them are related to cancer treatment. Particularly, ET is an attractive technique for cancer immunogene therapy because treatment of cells with electric pulses alone can induce immune responses to solid tumors, and the responses can be further enhanced by ET of plasmid DNA (pDNA) encoding therapeutic genes. Compared to other gene delivery methods, ET has several unique advantages. It is relatively inexpensive, flexible, and safe in clinical applications, and introduces only naked pDNA into cells without the use of additional chemicals or viruses. However, the efficiency of ET is still low, partly because biological mechanisms of ET in cells remain elusive. In previous studies, it was believed that pDNA entered the cells through transient pores created by electric pulses. As a result, the technique is commonly referred to as electroporation. However, recent discoveries have suggested that endocytosis plays an important role in cellular uptake and intracellular transport of electrotransfected pDNA. This review will discuss current progresses in the study of biological mechanisms underlying ET and future directions of research in this area. Understanding the mechanisms of pDNA transport in cells is critical for the development of new strategies for improving the efficiency of gene delivery in tumors.
Keywords: electrogene transfer; electroporation; electrotransfection; gene electroinjection; nonviral gene delivery.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures

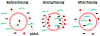
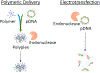

References
-
- Maggio I; Goncalves MA Genome editing at the crossroads of delivery, specificity, and fidelity. Trends Biotechnol 2015, 33 (5), 280–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

