Association of Amyloid Positron Emission Tomography With Changes in Diagnosis and Patient Treatment in an Unselected Memory Clinic Cohort: The ABIDE Project
- PMID: 29889941
- PMCID: PMC6143118
- DOI: 10.1001/jamaneurol.2018.1346
Association of Amyloid Positron Emission Tomography With Changes in Diagnosis and Patient Treatment in an Unselected Memory Clinic Cohort: The ABIDE Project
Abstract
Importance: Previous studies have evaluated the diagnostic effect of amyloid positron emission tomography (PET) in selected research cohorts. However, these research populations do not reflect daily practice, thus hampering clinical implementation of amyloid imaging.
Objective: To evaluate the association of amyloid PET with changes in diagnosis, diagnostic confidence, treatment, and patients' experiences in an unselected memory clinic cohort.
Design, setting, and participants: Amyloid PET using fluoride-18 florbetaben was offered to 866 patients who visited the tertiary memory clinic at the VU University Medical Center between January 2015 and December 2016 as part of their routine diagnostic dementia workup. Of these patients, 476 (55%) were included, 32 (4%) were excluded, and 358 (41%) did not participate. To enrich this sample, 31 patients with mild cognitive impairment from the University Medical Center Utrecht memory clinic were included. For each patient, neurologists determined a preamyloid and postamyloid PET diagnosis that existed of both a clinical syndrome (dementia, mild cognitive impairment, or subjective cognitive decline) and a suspected etiology (Alzheimer disease [AD] or non-AD), with a confidence level ranging from 0% to 100%. In addition, the neurologist determined patient treatment in terms of ancillary investigations, medication, and care. Each patient received a clinical follow-up 1 year after being scanned.
Main outcomes and measures: Primary outcome measures were post-PET changes in diagnosis, diagnostic confidence, and patient treatment.
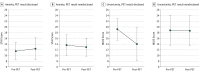
Results: Of the 507 patients (mean [SD] age, 65 (8) years; 201 women [39%]; mean [SD] Mini-Mental State Examination score, 25 [4]), 164 (32%) had AD dementia, 70 (14%) non-AD dementia, 114 (23%) mild cognitive impairment, and 159 (31%) subjective cognitive decline. Amyloid PET results were positive for 242 patients (48%). The suspected etiology changed for 125 patients (25%) after undergoing amyloid PET, more often due to a negative (82 of 265 [31%]) than a positive (43 of 242 [18%]) PET result (P < .01). Post-PET changes in suspected etiology occurred more frequently in patients older (>65 years) than younger (<65 years) than the typical age at onset of 65 years (74 of 257 [29%] vs 51 of 250 [20%]; P < .05). Mean diagnostic confidence (SD) increased from 80 (13) to 89 (13%) (P < .001). In 123 patients (24%), there was a change in patient treatment post-PET, mostly related to additional investigations and therapy.
Conclusions and relevance: This prospective diagnostic study provides a bridge between validating amyloid PET in a research setting and implementing this diagnostic tool in daily clinical practice. Both amyloid-positive and amyloid-negative results had substantial associations with changes in diagnosis and treatment, both in patients with and without dementia.
Conflict of interest statement
Figures

Comment in
-
Improving Evaluation of Patients With Cognitive Impairment With Amyloid Positron Emission Tomography.JAMA Neurol. 2018 Sep 1;75(9):1045-1046. doi: 10.1001/jamaneurol.2018.1010. JAMA Neurol. 2018. PMID: 29889928 No abstract available.
References
-
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184-185. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353-356. - PubMed
-
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306-319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

