Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial
- PMID: 29890086
- PMCID: PMC6221579
- DOI: 10.1164/rccm.201802-0215OC
Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial
Erratum in
-
Erratum: Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial.Am J Respir Crit Care Med. 2019 Mar 15;199(6):809. doi: 10.1164/rccm.v199erratum2. Am J Respir Crit Care Med. 2019. PMID: 30874457 Free PMC article. No abstract available.
Abstract
Rationale: New isolation of Pseudomonas aeruginosa (Pa) is generally treated with inhaled antipseudomonal antibiotics such as tobramycin inhalation solution (TIS). A therapeutic approach that complements traditional antimicrobial therapy by reducing the risk of pulmonary exacerbation and inflammation may ultimately prolong the time to Pa recurrence.
Objectives: To test the hypothesis that the addition of azithromycin to TIS in children with cystic fibrosis and early Pa decreases the risk of pulmonary exacerbation and prolongs the time to Pa recurrence.
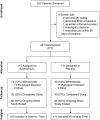
Methods: The OPTIMIZE (Optimizing Treatment for Early Pseudomonas aeruginosa Infection in Cystic Fibrosis) trial was a multicenter, double-blind, randomized, placebo-controlled, 18-month trial in children with CF, 6 months to 18 years of age, with early Pa. Azithromycin or placebo was given 3× weekly with standardized TIS.
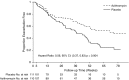
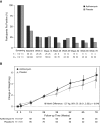
Measurements and main results: The primary endpoint was the time to pulmonary exacerbation requiring antibiotics and the secondary endpoint was the time to Pa recurrence, in addition to other clinical and safety outcomes. A total of 221 participants (111 placebo, 110 azithromycin) out of a planned 274 were enrolled. Enrollment was stopped early by the NHLBI because the trial had reached the prespecified interim boundary for efficacy. The risk of pulmonary exacerbation was reduced by 44% in the azithromycin group as compared with the placebo group (hazard ratio, 0.56; 95% confidence interval, 0.37-0.83; P = 0.004). Weight increased by 1.27 kg in the azithromycin group compared with the placebo group (95% confidence interval, 0.01-2.52; P = 0.046). No significant differences were seen in microbiological or other clinical or safety endpoints.
Conclusions: Azithromycin was associated with a significant reduction in the risk of pulmonary exacerbation and a sustained improvement in weight, but had no impact on microbiological outcomes in children with early Pa. Clinical trial registered with clinicaltrials.gov (NCT02054156).
Keywords: Pseudomonas aeruginosa; clinical trial; eradication; pulmonary exacerbation.
Figures

Comment in
-
Preservation of Lung Function in Cystic Fibrosis: Are Macrolides the Answer?Am J Respir Crit Care Med. 2018 Nov 1;198(9):1114-1116. doi: 10.1164/rccm.201806-1103ED. Am J Respir Crit Care Med. 2018. PMID: 30011225 No abstract available.
-
Reply to Shanthikumar et al.: Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis: Do the Benefits Outweigh the Harms?Am J Respir Crit Care Med. 2018 Nov 15;198(10):1349-1350. doi: 10.1164/rccm.201808-1462LE. Am J Respir Crit Care Med. 2018. PMID: 30138567 No abstract available.
-
Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis: Do the Benefits Outweigh the Harms?Am J Respir Crit Care Med. 2018 Nov 15;198(10):1348-1349. doi: 10.1164/rccm.201807-1329LE. Am J Respir Crit Care Med. 2018. PMID: 30138568 No abstract available.
References
-
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. - PubMed
-
- Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:75–81. - PubMed
-
- Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32:277–287. - PubMed
-
- Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151:134–139, 139.e1. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

