Pro-inflammatory response and hemostatic disorder induced by venom of the coral snake Micrurus tener tener IN C57BL/6 mice
- PMID: 29890232
- PMCID: PMC6440475
- DOI: 10.1016/j.toxicon.2018.06.063
Pro-inflammatory response and hemostatic disorder induced by venom of the coral snake Micrurus tener tener IN C57BL/6 mice
Abstract
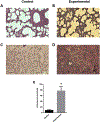
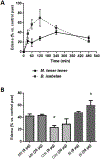
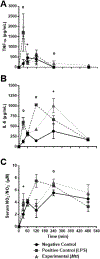
Micrurus venoms are known to induce mainly neurotoxicity in victims. However, other manifestations, including hemorrhage, edema, myotoxicity, complement activation, and hemostatic activity have been reported. In order to develop a more complete pharmacological profile of these venoms, inflammatory responses and hemostasis were evaluated in C57BL/6 mice treated with a sub-lethal dose of M. t. tener (Mtt) venom (8 μg/mouse), inoculated intraperitoneally. The venom induced moderate bleeding into the abdominal cavity and lungs, as well as infiltration of leukocytes into the liver. After 30 min, the release of pro-inflammatory mediators (TNF-α, IL-6, and NO) were observed, being most evident at 4 h. There was a decrease in hemoglobin and hematocrit levels at 72 h, a prolongation in coagulation times (PT and aPTT), a decrease in the fibrinogen concentration and an increase in fibrinolytic activity. In this animal model, it was proposed that Mtt venom induces inflammation with the release of mediators such as TNF-α, in response to the toxins. These mediators may activate hemostatic mechanisms, producing systemic fibrinolysis and hemorrhage. These findings suggest alternative treatments in Micrurus envenomations in which neurotoxic manifestations do not predominate.
Keywords: Edema; Hemostasis; Inflammation; Micrurus tener tener; Snake venom.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Contribution of endothelial cell and macrophage activation in the alterations induced by the venom of Micrurus tener tener in C57BL/6 mice.Mol Immunol. 2019 Dec;116:45-55. doi: 10.1016/j.molimm.2019.09.009. Epub 2019 Oct 7. Mol Immunol. 2019. PMID: 31600647 Free PMC article.
-
Hemostatic and toxinological diversities in venom of Micrurus tener tener, Micrurus fulvius fulvius and Micrurus isozonus coral snakes.Toxicon. 2011 Jul;58(1):35-45. doi: 10.1016/j.toxicon.2011.04.020. Epub 2011 May 8. Toxicon. 2011. PMID: 21596052 Free PMC article.
-
The Bold and the Beautiful: a Neurotoxicity Comparison of New World Coral Snakes in the Micruroides and Micrurus Genera and Relative Neutralization by Antivenom.Neurotox Res. 2017 Oct;32(3):487-495. doi: 10.1007/s12640-017-9771-4. Epub 2017 Jul 3. Neurotox Res. 2017. PMID: 28674788
-
Coral snake bites (Micrurus spp.) in Brazil: a review of literature reports.Clin Toxicol (Phila). 2016 Mar;54(3):222-34. doi: 10.3109/15563650.2015.1135337. Epub 2016 Jan 25. Clin Toxicol (Phila). 2016. PMID: 26808120 Review.
-
Unresolved issues in the understanding of the pathogenesis of local tissue damage induced by snake venoms.Toxicon. 2018 Jun 15;148:123-131. doi: 10.1016/j.toxicon.2018.04.016. Epub 2018 Apr 23. Toxicon. 2018. PMID: 29698755 Review.
Cited by
-
Micrurus lemniscatus venom stimulates leukocyte functions in vivo.Arch Toxicol. 2025 Apr;99(4):1591-1603. doi: 10.1007/s00204-025-03970-z. Epub 2025 Feb 13. Arch Toxicol. 2025. PMID: 39948207
-
Qualitative and Quantitative Study of the Changes in the Ultrastructure of Mammalian Adrenal Cortex Caused by the Venezuelan Tigra Mariposa (Bothrops venezuelensis) Snake Venom.J Microsc Ultrastruct. 2020 Sep 10;8(3):104-114. doi: 10.4103/JMAU.JMAU_49_19. eCollection 2020 Jul-Sep. J Microsc Ultrastruct. 2020. PMID: 33282685 Free PMC article.
-
Contribution of endothelial cell and macrophage activation in the alterations induced by the venom of Micrurus tener tener in C57BL/6 mice.Mol Immunol. 2019 Dec;116:45-55. doi: 10.1016/j.molimm.2019.09.009. Epub 2019 Oct 7. Mol Immunol. 2019. PMID: 31600647 Free PMC article.
-
Exploring the Safety of Pllans-II and Antitumoral Potential of Its Recombinant Isoform in Cervical Cancer Therapy.Cells. 2023 Dec 10;12(24):2812. doi: 10.3390/cells12242812. Cells. 2023. PMID: 38132131 Free PMC article.
References
-
- Aguilar I, Guerrero B, Salazar A, Girón M, Pérez J, Sánchez EE, Rodríguez-Acosta A, 2007. Individual venom variability in the South American rattlesnake Crotalus durissus cumanensis. Toxicon 50, 214–224. - PubMed
-
- Aird SD, 2002. Ophidian envenomation strategies and the role of purines. Toxicon 40, 335–393. - PubMed
-
- Arocha-Piñango CL, Marval E, Guerrero B, 2000. Lonomia genus caterpillar toxins: biochemical aspects. Biochimie 82, 937–942. - PubMed
-
- Barrios M, Rodríguez-Acosta A, Gil A, Salazar AM, Taylor P, Sánchez EE, Arocha-Piñango CL, Guerrero B, 2009. Comparative hemostatic parameters in BALB/c, C57BL/6 and C3H/He mice. Thromb. Res 124, 338–343. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

