A trial of type 12 purinergic (P2Y12) receptor inhibition with prasugrel identifies a potentially distinct endotype of patients with aspirin-exacerbated respiratory disease
- PMID: 29890239
- PMCID: PMC6286686
- DOI: 10.1016/j.jaci.2018.06.001
A trial of type 12 purinergic (P2Y12) receptor inhibition with prasugrel identifies a potentially distinct endotype of patients with aspirin-exacerbated respiratory disease
Abstract
Background: Aspirin-exacerbated respiratory disease (AERD) is characterized by asthma, recurrent nasal polyposis, and respiratory reactions on ingestion of COX-1 inhibitors. Increased numbers of platelet-leukocyte aggregates are present in the sinus tissue and blood of patients with AERD compared with that of aspirin-tolerant patients, and platelet activation can contribute to aspirin-induced reactions.
Objective: We sought to determine whether treatment with prasugrel, which inhibits platelet activation by blocking the type 12 purinergic (P2Y12) receptor, would attenuate the severity of sinonasal and respiratory symptoms induced during aspirin challenge in patients with AERD.
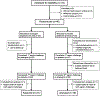
Methods: Forty patients with AERD completed a 10-week, double-blind, placebo-controlled crossover trial of prasugrel. All patients underwent oral aspirin challenges after 4 weeks of prasugrel and after 4 weeks of placebo. The primary outcome was a change in the provocative dose of aspirin that would elicit an increase in Total Nasal Symptom Score (TNSS) of 2 points. Changes in lung function, urinary eicosanoids, plasma tryptase, platelet-leukocyte aggregates, and platelet activation were also recorded.
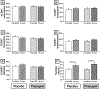
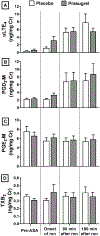
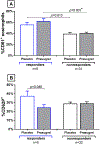
Results: Prasugrel did not significantly change the mean increase in TNSS of 2 points (79 ± 15 for patients receiving placebo and 139 ± 32 for patients receiving prasugrel, P = .10), platelet-leukocyte aggregates, or increases in urinary leukotriene E4 and prostaglandin D2 metabolite levels during aspirin-induced reactions in the study population as a whole. Five subjects (responders) reacted to aspirin while receiving placebo but did not have any reaction to aspirin challenge after the prasugrel arm. In contrast to prasugrel nonresponders (35 subjects), the prasugrel responders had smaller reaction-induced increases in TNSS; did not have significant aspirin-induced increases in urinary leukotriene E4, prostaglandin D2 metabolite, or thromboxane B2 levels; and did not display increases in serum tryptase levels during aspirin reactions on the placebo arm, all of which were observed in the nonresponders.
Conclusion: In the overall study population, prasugrel did not attenuate aspirin-induced symptoms, possibly because it failed to decrease the frequencies of platelet-adherent leukocytes or to diminish aspirin-induced mast cell activation. In a small subset of patients with AERD who had greater baseline platelet activation and milder upper respiratory symptoms during aspirin-induced reactions, P2Y12 receptor antagonism with prasugrel completely inhibited all aspirin-induced reaction symptoms, suggesting a contribution from P2Y12 receptor signaling in this subset.
Keywords: Aspirin-exacerbated respiratory disease; NSAID-exacerbated respiratory disease; P2Y(12); Samter triad; double-blind; leukotrienes; placebo-controlled crossover trial; prasugrel; randomized.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest: T Laidlaw has served on scientific advisory boards for GlaxoSmithKline and Allakos and has received consultancy fees from Knopp Biosciences and Sanofi-Genzyme. K Cahill has served on scientific advisory boards for Teva. J Cardet, K Murphy, J Cui, B Dioneda, P Kothari, B Raby, and J Boyce have no conflicts of interest to disclose. E Israel has received consultancy fees from AstraZeneca, Philips Respironics, Regeneron Pharmaceuticals, Birk Rock Bio, Nuvelution Pharmaceuticals, Vitaeris Inc, Sanofi, and Merck.
Figures



Similar articles
-
Trial of thromboxane receptor inhibition with ifetroban: TP receptors regulate eicosanoid homeostasis in aspirin-exacerbated respiratory disease.J Allergy Clin Immunol. 2023 Sep;152(3):700-710.e3. doi: 10.1016/j.jaci.2023.03.030. Epub 2023 Apr 15. J Allergy Clin Immunol. 2023. PMID: 37068712 Free PMC article.
-
Unique Effect of Aspirin Therapy on Biomarkers in Aspirin-exacerbated Respiratory Disease. A Prospective Trial.Am J Respir Crit Care Med. 2019 Sep 15;200(6):704-711. doi: 10.1164/rccm.201809-1755OC. Am J Respir Crit Care Med. 2019. PMID: 30978291 Free PMC article.
-
Real-time dose adjustment using point-of-care platelet reactivity testing in a double-blind study of prasugrel in children with sickle cell anaemia.Thromb Haemost. 2017 Feb 28;117(3):580-588. doi: 10.1160/TH16-09-0731. Epub 2016 Dec 8. Thromb Haemost. 2017. PMID: 27929203 Clinical Trial.
-
Platelets in patients with aspirin-exacerbated respiratory disease.J Allergy Clin Immunol. 2015 Jun;135(6):1407-14; quiz 1415. doi: 10.1016/j.jaci.2015.02.005. J Allergy Clin Immunol. 2015. PMID: 26051947 Free PMC article. Review.
-
Effect of ticagrelor versus prasugrel on platelet reactivity: a meta-analysis.Coron Artery Dis. 2017 Nov;28(7):597-604. doi: 10.1097/MCA.0000000000000541. Coron Artery Dis. 2017. PMID: 28968313 Review.
Cited by
-
Lipid-mediated innate lymphoid cell recruitment and activation in aspirin-exacerbated respiratory disease.Ann Allergy Asthma Immunol. 2021 Feb;126(2):135-142. doi: 10.1016/j.anai.2020.09.011. Epub 2020 Sep 17. Ann Allergy Asthma Immunol. 2021. PMID: 32950684 Free PMC article. Review.
-
Plasma tryptase elevation during aspirin-induced reactions in aspirin-exacerbated respiratory disease.J Allergy Clin Immunol. 2019 Feb;143(2):799-803.e2. doi: 10.1016/j.jaci.2018.10.007. Epub 2018 Oct 16. J Allergy Clin Immunol. 2019. PMID: 30339852 Free PMC article.
-
Biomarkers for predicting response to aspirin therapy in aspirin-exacerbated respiratory disease.Clin Exp Allergy. 2021 Aug;51(8):1046-1056. doi: 10.1111/cea.13886. Epub 2021 May 11. Clin Exp Allergy. 2021. PMID: 33905579 Free PMC article.
-
Rapid and sustained effect of dupilumab on clinical and mechanistic outcomes in aspirin-exacerbated respiratory disease.J Allergy Clin Immunol. 2022 Aug;150(2):415-424. doi: 10.1016/j.jaci.2022.04.007. Epub 2022 Apr 20. J Allergy Clin Immunol. 2022. PMID: 35460728 Free PMC article.
-
Mediator production and severity of aspirin-induced respiratory reactions: Impact of sampling site and body mass index.J Allergy Clin Immunol. 2022 Jul;150(1):170-177.e6. doi: 10.1016/j.jaci.2021.12.787. Epub 2022 Jan 11. J Allergy Clin Immunol. 2022. PMID: 35026207 Free PMC article.
References
-
- Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol 2015;135(3):676–81 e1. - PubMed
-
- Israel E, Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Shapiro J, et al. The pivotal role of 5-lipoxygenase products in the reaction of aspirin-sensitive asthmatics to aspirin. Amer Rev Resp Dis 1993;148(6 Pt 1):1447–51. - PubMed
-
- Yoshida S, Amayasu H, Sakamoto H, Onuma K, Shoji T, Nakagawa H, et al. Cromolyn sodium prevents bronchoconstriction and urinary LTE4 excretion in aspirin-induced asthma. Ann Allergy Asthma 1998;80(2):171–6. - PubMed
-
- Maclouf J, Antoine C, Henson PM, Murphy RC. Leukotriene C4 formation by transcellular biosynthesis. Ann N Y Acad Sci 1994;714:143–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL128241/HL/NHLBI NIH HHS/United States
- R01 HL117945/HL/NHLBI NIH HHS/United States
- T32 AI007306/AI/NIAID NIH HHS/United States
- U19 AI095219/AI/NIAID NIH HHS/United States
- K23 AI125785/AI/NIAID NIH HHS/United States
- R01 AI078908/AI/NIAID NIH HHS/United States
- K23 HL111113/HL/NHLBI NIH HHS/United States
- R56 AI052353/AI/NIAID NIH HHS/United States
- R01 HL136209/HL/NHLBI NIH HHS/United States
- R37 AI052353/AI/NIAID NIH HHS/United States
- R01 AI136041/AI/NIAID NIH HHS/United States
- K23 AI118804/AI/NIAID NIH HHS/United States
- R01 AI052353/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

