N-acetylcysteine prevents ketamine-induced adverse effects on development, heart rate and monoaminergic neurons in zebrafish
- PMID: 29890257
- PMCID: PMC6102060
- DOI: 10.1016/j.neulet.2018.06.014
N-acetylcysteine prevents ketamine-induced adverse effects on development, heart rate and monoaminergic neurons in zebrafish
Abstract
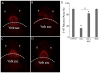
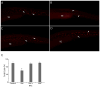
N-acetylcysteine, a precursor molecule of glutathione, is an antioxidant. Ketamine, a pediatric anesthetic, has been implicated in cardiotoxicity and neurotoxicity including modulation of monoaminergic systems in mammals and zebrafish. Here, we show that N-acetylcysteine prevents ketamine's adverse effects on development and monoaminergic neurons in zebrafish embryos. The effects of ketamine and N-acetylcysteine alone or in combination were measured on the heart rate, body length, brain serotonergic neurons and tyrosine hydroxylase-immunoreactive (TH-IR) neurons. In the absence of N-acetylcysteine, a concentration of ketamine that produces an internal embryo exposure level comparable to human anesthetic plasma concentrations significantly reduced heart rate and body length and those effects were prevented by N-acetylcysteine co-treatment. Ketamine also reduced the areas occupied by serotonergic neurons in the brain, whereas N-acetylcysteine co-exposure counteracted this effect. TH-IR neurons in the embryo brain and TH-IR cells in the trunk were significantly reduced with ketamine treatment, but not in the presence of N-acetylcysteine. In our continued search for compounds that can prevent ketamine toxicity, this study using specific endpoints of developmental toxicity, cardiotoxicity and neurotoxicity, demonstrates protective effects of N-acetylcysteine against ketamine's adverse effects. This is the first study that shows the protective effects of N-acetylcysteine on ketamine-induced developmental defects of monoaminergic neurons as observed in a whole organism.
Keywords: 5-HT; Developmental toxicity; Ketamine; N-acetyl cysteine; Tyrosine hydroxylase; Zebrafish.
Published by Elsevier B.V.
Figures



Similar articles
-
N-acetylcysteine prevents verapamil-induced cardiotoxicity with no effect on the noradrenergic arch-associated neurons in zebrafish.Food Chem Toxicol. 2020 Oct;144:111559. doi: 10.1016/j.fct.2020.111559. Epub 2020 Jul 5. Food Chem Toxicol. 2020. PMID: 32640352
-
Acetyl L-carnitine targets adenosine triphosphate synthase in protecting zebrafish embryos from toxicities induced by verapamil and ketamine: An in vivo assessment.J Appl Toxicol. 2017 Feb;37(2):192-200. doi: 10.1002/jat.3340. Epub 2016 May 18. J Appl Toxicol. 2017. PMID: 27191126 Free PMC article.
-
Morphological and behavioral responses of zebrafish after 24h of ketamine embryonic exposure.Toxicol Appl Pharmacol. 2017 Apr 15;321:27-36. doi: 10.1016/j.taap.2017.02.013. Epub 2017 Feb 17. Toxicol Appl Pharmacol. 2017. PMID: 28215996
-
Relationship between ketamine-induced developmental neurotoxicity and NMDA receptor-mediated calcium influx in neural stem cell-derived neurons.Neurotoxicology. 2017 May;60:254-259. doi: 10.1016/j.neuro.2016.04.015. Epub 2016 Apr 27. Neurotoxicology. 2017. PMID: 27132109 Review.
-
Zebrafish (Danio rerio): A potential model to assess developmental toxicity of ketamine.Chemosphere. 2022 Mar;291(Pt 3):133033. doi: 10.1016/j.chemosphere.2021.133033. Epub 2021 Nov 22. Chemosphere. 2022. PMID: 34822872 Review.
Cited by
-
Esketamine Exposure Impairs Cardiac Development and Function in Zebrafish Larvae.Toxics. 2024 Jun 13;12(6):427. doi: 10.3390/toxics12060427. Toxics. 2024. PMID: 38922107 Free PMC article.
-
Neuronal and peripheral damages induced by synthetic psychoactive substances: an update of recent findings from human and animal studies.Neural Regen Res. 2020 May;15(5):802-816. doi: 10.4103/1673-5374.268895. Neural Regen Res. 2020. PMID: 31719240 Free PMC article. Review.
-
Nifedipine toxicity is exacerbated by acetyl l-carnitine but alleviated by low-dose ketamine in zebrafish in vivo.J Appl Toxicol. 2020 Feb;40(2):257-269. doi: 10.1002/jat.3901. Epub 2019 Oct 9. J Appl Toxicol. 2020. PMID: 31599005 Free PMC article.
-
Hypoplasia of dopaminergic neurons by hypoxia-induced neurotoxicity is associated with disrupted swimming development of larval zebrafish.Front Cell Neurosci. 2022 Sep 23;16:963037. doi: 10.3389/fncel.2022.963037. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36212692 Free PMC article.
-
Ketamine-induced attenuation of reactive oxygen species in zebrafish is prevented by acetyl l-carnitine in vivo.Neurosci Lett. 2019 Jul 27;706:36-42. doi: 10.1016/j.neulet.2019.05.009. Epub 2019 May 9. Neurosci Lett. 2019. PMID: 31078678 Free PMC article.
References
-
- Rhodes K, Braakhuis A. Performance and Side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med. 2017;47:1619–1636. - PubMed
-
- Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation, Update. Ann Emerg Med. 2011;57(2011):449–461. - PubMed
-
- Wang C, Liu F, Patterson TA, Paule MG, Slikker W., Jr Relationship between ketamine-induced developmental neurotoxicity and NMDA receptor-mediated calcium influx in neural stem cell-derived neurons. Neurotoxicology. 2017;60:254–259. - PubMed
-
- Tadler SC, Mickey BJ. Emerging evidence for antidepressant actions of anesthetic agents. Curr Opin Anaesthesiol. 2018 (in press) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

