Cobalt Complex with Thiazole-Based Ligand as New Pseudomonas aeruginosa Quorum Quencher, Biofilm Inhibitor and Virulence Attenuator
- PMID: 29890626
- PMCID: PMC6099793
- DOI: 10.3390/molecules23061385
Cobalt Complex with Thiazole-Based Ligand as New Pseudomonas aeruginosa Quorum Quencher, Biofilm Inhibitor and Virulence Attenuator
Abstract
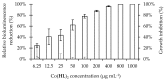
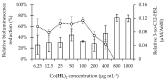
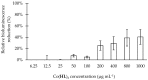
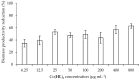
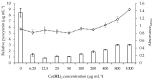
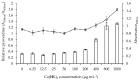
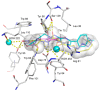
Pseudomonas aeruginosa is one of the most dreaded human pathogens, because of its intrinsic resistance to a number of commonly used antibiotics and ability to form sessile communities (biofilms). Innovative treatment strategies are required and that can rely on the attenuation of the pathogenicity and virulence traits. The interruption of the mechanisms of intercellular communication in bacteria (quorum sensing) is one of such promising strategies. A cobalt coordination compound (Co(HL)₂) synthesized from (E)-2-(2-(pyridin-2-ylmethylene)hydrazinyl)-4-(p-tolyl)thiazole (HL) is reported herein for the first time to inhibit P. aeruginosa 3-oxo-C12-HSL-dependent QS system (LasI/LasR system) and underling phenotypes (biofilm formation and virulence factors). Its interactions with a possible target, the transcriptional activator protein complex LasR-3-oxo-C12-HSL, was studied by molecular modeling with the coordination compound ligand having stronger predicted interactions than those of co-crystallized ligand 3-oxo-C12-HSL, as well as known-binder furvina. Transition metal group 9 coordination compounds may be explored in antipathogenic/antibacterial drug design.
Keywords: antibacterial resistance; antivirulence/antipathogenic compounds; biofilm prevention; cobalt complex; furvina; pyocyanin; pyoverdine; quorum sensing inhibition; transcriptional activator protein LasR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Furvina inhibits the 3-oxo-C12-HSL-based quorum sensing system of Pseudomonas aeruginosa and QS-dependent phenotypes.Biofouling. 2017 Feb;33(2):156-168. doi: 10.1080/08927014.2017.1280732. Epub 2017 Jan 31. Biofouling. 2017. PMID: 28140677
-
Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model.PLoS One. 2017 Apr 28;12(4):e0176883. doi: 10.1371/journal.pone.0176883. eCollection 2017. PLoS One. 2017. PMID: 28453568 Free PMC article.
-
Inhibition of biofilm formation, quorum sensing activity and molecular docking study of isolated 3, 5, 7-Trihydroxyflavone from Alstonia scholaris leaf against P.aeruginosa.Bioorg Chem. 2019 Jun;87:291-301. doi: 10.1016/j.bioorg.2019.03.050. Epub 2019 Mar 18. Bioorg Chem. 2019. PMID: 30913464
-
Niclosamide as a potential antivirulence agent disrupting quorum sensing in Pseudomonas aeruginosa: A molecular and in silico approach.Biochem Biophys Res Commun. 2025 May 5;762:151742. doi: 10.1016/j.bbrc.2025.151742. Epub 2025 Apr 3. Biochem Biophys Res Commun. 2025. PMID: 40199129
-
Sodium ascorbate as a quorum sensing inhibitor of Pseudomonas aeruginosa.J Appl Microbiol. 2014 Nov;117(5):1388-99. doi: 10.1111/jam.12631. Epub 2014 Oct 3. J Appl Microbiol. 2014. PMID: 25175797
Cited by
-
It is the time for quorum sensing inhibition as alternative strategy of antimicrobial therapy.Cell Commun Signal. 2023 Jun 14;21(1):133. doi: 10.1186/s12964-023-01154-9. Cell Commun Signal. 2023. PMID: 37316831 Free PMC article. Review.
-
Posttranslational chemical installation of azoles into translated peptides.Nat Commun. 2021 Jan 29;12(1):696. doi: 10.1038/s41467-021-20992-0. Nat Commun. 2021. PMID: 33514734 Free PMC article.
-
Quantitative mapping of mRNA 3' ends in Pseudomonas aeruginosa reveals a pervasive role for premature 3' end formation in response to azithromycin.PLoS Genet. 2021 Jul 12;17(7):e1009634. doi: 10.1371/journal.pgen.1009634. eCollection 2021 Jul. PLoS Genet. 2021. PMID: 34252072 Free PMC article.
-
Growing emergence of drug-resistant Pseudomonas aeruginosa and attenuation of its virulence using quorum sensing inhibitors: A critical review.Iran J Basic Med Sci. 2021 Jun;24(6):699-719. doi: 10.22038/IJBMS.2021.49151.11254. Iran J Basic Med Sci. 2021. PMID: 34630947 Free PMC article. Review.
-
Innovative application of ceftriaxone as a quorum sensing inhibitor in Pseudomonas aeruginosa.Sci Rep. 2025 Feb 11;15(1):5022. doi: 10.1038/s41598-025-87609-0. Sci Rep. 2025. PMID: 39934154 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

