An Overview of Biotransformation and Toxicity of Diterpenes
- PMID: 29890639
- PMCID: PMC6100218
- DOI: 10.3390/molecules23061387
An Overview of Biotransformation and Toxicity of Diterpenes
Abstract
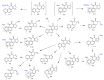
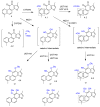
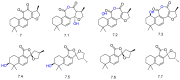
Diterpenes have been identified as active compounds in several medicinal plants showing remarkable biological activities, and some isolated diterpenes are produced at commercial scale to be used as medicines, food additives, in the synthesis of fragrances, or in agriculture. There is great interest in developing methods to obtain derivatives of these compounds, and biotransformation processes are interesting tools for the structural modification of natural products with complex chemical structures. Biotransformation processes also have a crucial role in drug development and/or optimization. The understanding of the metabolic pathways for both phase I and II biotransformation of new drug candidates is mandatory for toxicity and efficacy evaluation and part of preclinical studies. This review presents an overview of biotransformation processes of diterpenes carried out by microorganisms, plant cell cultures, animal and human liver microsomes, and rats, chickens, and swine in vivo and highlights the main enzymatic reactions involved in these processes and the role of diterpenes that may be effectively exploited by other fields.
Keywords: biotransformation; diterpenes; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
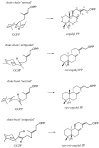
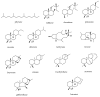
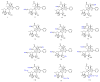
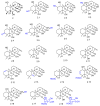

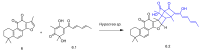
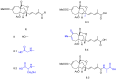
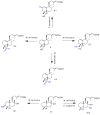
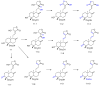


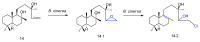
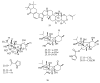
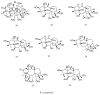

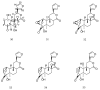
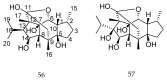
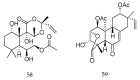
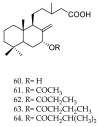
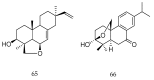
References
-
- Mafu S., Zerbe P. Plant diterpenoid metabolism for manufacturing the biopharmaceuticals of tomorrow: Prospects and challenges. Phytochem. Rev. 2018;17:113–130. doi: 10.1007/s11101-017-9513-5. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

