Improved Information and Educational Messages on Outer Packaging of Micronutrient Powders Distributed in Indonesia Increase Caregiver Knowledge and Adherence to Recommended Use
- PMID: 29890670
- PMCID: PMC6024872
- DOI: 10.3390/nu10060747
Improved Information and Educational Messages on Outer Packaging of Micronutrient Powders Distributed in Indonesia Increase Caregiver Knowledge and Adherence to Recommended Use
Abstract
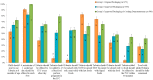
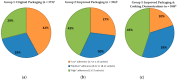
The objective of this study was to examine the influence of improved information and educational messages on outer packaging of a micronutrient powder (MNP), locally known as “Taburia”, on knowledge and adherence to recommended use. A community-based cluster randomized controlled trial was conducted among 1149 caregivers and their children aged 6⁻36 months. Caregiver⁻child dyads were randomized by their villages to receive 30 sachets of Taburia with the: (i) original outer packaging; (ii) improved outer packaging; or (iii) improved outer packaging combined with cooking demonstrations. Adherence to Taburia use was assessed through caregiver interviews and observation of unused sachets during home visits; “high” adherence was defined as consuming 13⁻17 sachets in the previous month. Data collection included surveys and focus groups discussions. The majority of caregivers (>80%) preferred the improved packaging because it was more attractive and contained more comprehensive information. Caregivers who received the improved packaging had better knowledge regarding the recommended use of Taburia (p < 0.001) and higher adherence with the prescribed use of Taburia (43% with “high” adherence) (p < 0.001) than those who received the original packaging (29% with “high” adherence). Caregivers who participated in cooking demonstrations generally had better knowledge regarding the benefits of Taburia and recommended use, but this did not lead to higher adherence to recommended use. “Underconsumption” of Taburia (≤7 sachets) was much less prevalent than “overconsumption” (≥23 sachets), and original packaging users were more likely to consume Taburia daily instead of every two days as recommended. We conclude that the design of the outer packaging and comprehensiveness of information provided are important influencers of recommended MNP use by caregivers.
Keywords: Indonesia; adherence; infants and young children; micronutrient powders; packaging.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures


References
-
- International Food Policy Research Institute . Global Nutrition Report 2016: From Promise to Impact: Ending Malnutrition by 2030. International Food Policy Research Institute; Washington, DC, USA: 2016.
-
- National Institute of Health Research and Development (NIHRD) Ministry of Health (MOH) Indonesia . Indonesia Basic Health Research 2013. Book 1. NIHRD & MOH; Jakarta, Indonesia: 2014.
-
- National Population and Family Planning Board. Central Bureau of Statistics. Ministry of Health Indonesia . Indonesia Demographic and Health Survey 2012. Central Bureau of Statistics; Jakarta, Indonesia: 2013. Measure DHS ICF International.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

