Early Life Vitamin C Deficiency Does Not Alter Morphology of Hippocampal CA1 Pyramidal Neurons or Markers of Synaptic Plasticity in a Guinea Pig Model
- PMID: 29890692
- PMCID: PMC6024653
- DOI: 10.3390/nu10060749
Early Life Vitamin C Deficiency Does Not Alter Morphology of Hippocampal CA1 Pyramidal Neurons or Markers of Synaptic Plasticity in a Guinea Pig Model
Abstract
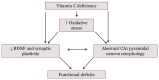
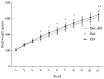
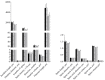
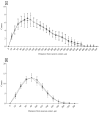
Approximately 15% of the Western world population, including pregnant women and their children, is characterized as vitamin C (vitC) deficient. In guinea pigs, early life vitC deficiency causes spatial memory deficits, decreased hippocampal volume and neuron numbers, in otherwise clinically healthy animals. We hypothesized that vitC deficiency leads to decreased brain-derived neurotrophic factor and synaptic plasticity markers in selected brain areas (frontal cortex, hippocampus and striatum) and cause morphological changes in cornu ammonis 1 pyramidal neurons of the hippocampus either through a direct effect or indirectly by increased oxidative stress. Fifty-seven female guinea pigs were allocated to three groups receiving either 1390, 100 or 0⁻50 mg vitC/kg feed for 11 weeks. Dietary vitC levels were reflected in the plasma, cortical and adrenal gland levels, however, redox imbalance was only present in the adrenal glands allowing for the investigation of a direct influence of vitC deficiency on the chosen parameters in the brain. Synaptic plasticity markers were not affected in the investigated brain areas and no differences in isolated pyramidal neuron morphology was recorded. Based on our findings, it appears that vitC deficiency may primarily elicit impaired neuronal function through increased levels of oxidative stress.
Keywords: cavia porcellus; neuronal morphology; synaptic plasticity; vitamin C deficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
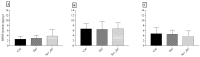
References
-
- Tolsa C.B., Zimine S., Warfield S.K., Freschi M., Rossignol A.S., Lazeyras F., Hanquinet S., Pfizenmaier M., Hüppi P.S. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr. Res. 2004;56:132–138. doi: 10.1203/01.PDR.0000128983.54614.7E. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

