New Tetrahydroisoquinoline Derivatives Overcome Pgp Activity in Brain-Blood Barrier and Glioblastoma Multiforme in Vitro
- PMID: 29890725
- PMCID: PMC6099747
- DOI: 10.3390/molecules23061401
New Tetrahydroisoquinoline Derivatives Overcome Pgp Activity in Brain-Blood Barrier and Glioblastoma Multiforme in Vitro
Abstract
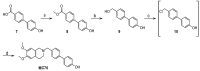
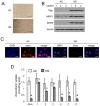
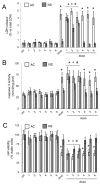
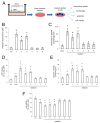
P-glycoprotein (Pgp) determines resistance to a broad spectrum of drugs used against glioblastoma multiforme (GB). Indeed, Pgp is highly expressed in GB stem cells and in the brain-blood barrier (BBB), the peculiar endothelium surrounding the brain. Inhibiting Pgp activity in the BBB and GB is still an open challenge. Here, we tested the efficacy of a small library of tetrahydroisoquinoline derivatives with an EC50 for Pgp ≤ 50 nM, in primary human BBB cells and in patient-derived GB samples, from which we isolated differentiated/adherent cells (AC, i.e., Pgp-negative/doxorubicin-sensitive cells) and stem cells (neurospheres, NS, i.e., Pgp-positive/doxorubicin-resistant cells). Three compounds used at 1 nM increased the delivery of doxorubicin, a typical substrate of Pgp, across BBB monolayer, without altering the expression and activity of other transporters. The compounds increased the drug accumulation within NS, restoring doxorubicin-induced necrosis and apoptosis, and reducing cell viability. In co-culture systems, the compounds added to the luminal face of BBB increased the delivery of doxorubicin to NS growing under BBB and rescued the drug’s cytotoxicity. Our work identified new ligands of Pgp active at low nanomolar concentrations. These compounds reduce Pgp activity in BBB and GB and improve in vitro chemotherapy efficacy in this tumor.
Keywords: P-glycoprotein; brain-blood barrier; doxorubicin; glioblastoma multiforme.
Conflict of interest statement
The authors declare there are no conflict of interest.
Figures


Similar articles
-
Validation of Thiosemicarbazone Compounds as P-Glycoprotein Inhibitors in Human Primary Brain-Blood Barrier and Glioblastoma Stem Cells.Mol Pharm. 2019 Aug 5;16(8):3361-3373. doi: 10.1021/acs.molpharmaceut.9b00018. Epub 2019 Jul 2. Mol Pharm. 2019. PMID: 31265310
-
Efflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor model.J Pharmacol Exp Ther. 2015 Nov;355(2):264-71. doi: 10.1124/jpet.115.228213. Epub 2015 Sep 9. J Pharmacol Exp Ther. 2015. PMID: 26354993 Free PMC article.
-
Temozolomide downregulates P-glycoprotein expression in glioblastoma stem cells by interfering with the Wnt3a/glycogen synthase-3 kinase/β-catenin pathway.Neuro Oncol. 2013 Nov;15(11):1502-17. doi: 10.1093/neuonc/not104. Epub 2013 Jul 28. Neuro Oncol. 2013. PMID: 23897632 Free PMC article.
-
Radioligands targeting P-glycoprotein and other drug efflux proteins at the blood-brain barrier.J Labelled Comp Radiopharm. 2013 Mar-Apr;56(3-4):68-77. doi: 10.1002/jlcr.2993. J Labelled Comp Radiopharm. 2013. PMID: 24285312 Review.
-
P-glycoprotein-mediated efflux transport of anticancer drugs at the blood-brain barrier.Ther Drug Monit. 1998 Oct;20(5):588-90. doi: 10.1097/00007691-199810000-00024. Ther Drug Monit. 1998. PMID: 9780140 Review.
Cited by
-
Multi-Targeting Approach in Glioblastoma Using Computer-Assisted Drug Discovery Tools to Overcome the Blood-Brain Barrier and Target EGFR/PI3Kp110β Signaling.Cancers (Basel). 2022 Jul 19;14(14):3506. doi: 10.3390/cancers14143506. Cancers (Basel). 2022. PMID: 35884571 Free PMC article.
-
Targeted Self-Emulsifying Drug Delivery Systems to Restore Docetaxel Sensitivity in Resistant Tumors.Pharmaceutics. 2022 Jan 26;14(2):292. doi: 10.3390/pharmaceutics14020292. Pharmaceutics. 2022. PMID: 35214025 Free PMC article.
-
Estrogen Receptors as Molecular Targets of Endocrine Therapy for Glioblastoma.Int J Mol Sci. 2021 Nov 17;22(22):12404. doi: 10.3390/ijms222212404. Int J Mol Sci. 2021. PMID: 34830286 Free PMC article. Review.
-
Tetrahydroquinoline/4,5-Dihydroisoxazole Molecular Hybrids as Inhibitors of Breast Cancer Resistance Protein (BCRP/ABCG2).ChemMedChem. 2021 Sep 6;16(17):2686-2694. doi: 10.1002/cmdc.202100188. Epub 2021 May 18. ChemMedChem. 2021. PMID: 33844464 Free PMC article.
-
Thermomagnetic Resonance Effect of the Extremely Low Frequency Electromagnetic Field on Three-Dimensional Cancer Models.Int J Mol Sci. 2022 Jul 19;23(14):7955. doi: 10.3390/ijms23147955. Int J Mol Sci. 2022. PMID: 35887313 Free PMC article.
References
-
- Salmaggi A., Boiardi A., Gelati M., Russo A., Calatozzolo C., Ciusani E., Sciacca F.L., Ottolina A., Parati E.A., La Porta C., et al. GBM-Derived Tumorospheres Identify a Population of Tumor Stem-Like Cells with Angiogenic Potential and Enhanced Multidrug Resistance Phenotype. Glia. 2006;54:850–860. doi: 10.1002/glia.20414. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

