The Impact of the Nitric Oxide (NO)/Soluble Guanylyl Cyclase (sGC) Signaling Cascade on Kidney Health and Disease: A Preclinical Perspective
- PMID: 29890734
- PMCID: PMC6032334
- DOI: 10.3390/ijms19061712
The Impact of the Nitric Oxide (NO)/Soluble Guanylyl Cyclase (sGC) Signaling Cascade on Kidney Health and Disease: A Preclinical Perspective
Abstract
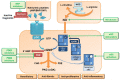
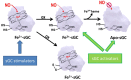
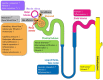
Chronic Kidney Disease (CKD) is a highly prevalent disease with a substantial medical need for new and more efficacious treatments. The Nitric Oxide (NO), soluble guanylyl cyclase (sGC), cyclic guanosine monophosphate (cGMP) signaling cascade regulates various kidney functions. cGMP directly influences renal blood flow, renin secretion, glomerular function, and tubular exchange processes. Downregulation of NO/sGC/cGMP signaling results in severe kidney pathologies such as CKD. Therefore, treatment strategies aiming to maintain or increase cGMP might have beneficial effects for the treatment of progressive kidney diseases. Within this article, we review the NO/sGC/cGMP signaling cascade and its major pharmacological intervention sites. We specifically focus on the currently known effects of cGMP on kidney function parameters. Finally, we summarize the preclinical evidence for kidney protective effects of NO-donors, PDE inhibitors, sGC stimulators, and sGC activators.
Keywords: cGMP; chronic kidney disease; nitric oxide; sGC; sGC activator; sGC stimulator; soluble guanylyl cyclase.
Conflict of interest statement
All authors are full-time employees of Bayer AG, Pharmaceuticals.
Figures
References
-
- Rahaman M.M., Nguyen A.T., Miller M.P., Hahn S.A., Sparacino-Watkins C., Jobbagy S., Carew N.T., Cantu-Medellin N., Wood K.C., Baty C.J., et al. Cytochrome b5 reductase 3 modulates soluble guanylate cyclase redox state and cgmp signaling. Circ. Res. 2017;121:137–148. doi: 10.1161/CIRCRESAHA.117.310705. - DOI - PMC - PubMed
-
- Sasser J.M., Brinson K.N., Tipton A.J., Crislip G.R., Sullivan J.C. Blood pressure, sex, and female sex hormones influence renal inner medullary nitric oxide synthase activity and expression in spontaneously hypertensive rats. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001738. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

