Antiviral Effects of Clinically-Relevant Interferon-α and Ribavirin Regimens against Dengue Virus in the Hollow Fiber Infection Model (HFIM)
- PMID: 29890736
- PMCID: PMC6024321
- DOI: 10.3390/v10060317
Antiviral Effects of Clinically-Relevant Interferon-α and Ribavirin Regimens against Dengue Virus in the Hollow Fiber Infection Model (HFIM)
Abstract
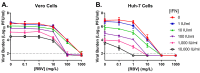
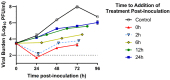
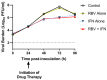
Dengue virus (DENV) is the most prevalent mosquito-borne viral illness in humans. Currently, there are no therapeutic agents available to prevent or treat DENV infections. Our objective was to fill this unmet medical need by evaluating the antiviral activity of interferon-α (IFN) and ribavirin (RBV) as a combination therapy against DENV. DENV-infected Vero and Huh-7 cells were exposed to RBV and/or IFN, and the viral burden was quantified over time by plaque assay. Drug-drug interactions for antiviral effect were determined by fitting a mathematical model to the data. We then assessed clinically-relevant exposures of IFN plus RBV using the hollow fiber infection model (HFIM) system. RBV monotherapy was only effective against DENV at toxic concentrations in Vero and Huh-7 cells. IFN, as a single agent, did inhibit DENV replication at physiological concentrations and viral suppression was substantial in Huh-7 cells (Half maximal effective concentration (EC50) = 58.34 IU/mL). As a combination therapy, RBV plus IFN was additive for viral suppression in both cell lines; however, enhancement of antiviral activity at clinically-achievable concentrations was observed only in Huh-7 cells. Finally, clinical exposures of RBV plus IFN suppressed DENV replication by 99% even when treatment was initiated 24 h post-infection in the HFIM. Further evaluation revealed that the antiviral effectiveness of the combination regimen against DENV is mostly attributed to activity associated with IFN. These findings suggest that IFN is a potential therapeutic strategy for the treatment of DENV.
Keywords: Dengue virus; antiviral therapy; combination therapy; hollow fiber infection model; interferon-α; mathematical modeling; ribavirin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

