Optimized Adeno-Associated Viral-Mediated Human Factor VIII Gene Therapy in Cynomolgus Macaques
- PMID: 29890905
- PMCID: PMC12199626
- DOI: 10.1089/hum.2018.080
Optimized Adeno-Associated Viral-Mediated Human Factor VIII Gene Therapy in Cynomolgus Macaques
Abstract
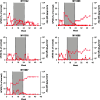
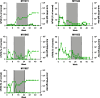
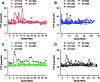
Hemophilia A is a common hereditary bleeding disorder that is characterized by a deficiency of human blood coagulation factor VIII (hFVIII). Previous studies with adeno-associated viral (AAV) vectors identified two liver-specific promoter and enhancer combinations (E03.TTR and E12.A1AT) that drove high level expression of a codon-optimized, B-domain-deleted hFVIII transgene in a mouse model of the disease. This study further evaluated these enhancer/promoter combinations in cynomolgus macaques using two different AAV capsids (AAVrh10 and AAVhu37). Each of the four vector combinations was administered intravenously at a dose of 1.2 × 1013 genome copy/kg into five macaques per group. Delivery of the hFVIII transgene via the AAVhu37 capsid resulted in a substantial increase in hFVIII expression compared to animals administered with AAVrh10 vectors. Two weeks after administration of E03.TTR packaged within the AAVhu37 capsid, average hFVIII expression was 20.2 ± 5.0% of normal, with one animal exhibiting peak expression of 37.1% of normal hFVIII levels. The majority of animals generated an anti-hFVIII antibody response by week 8-10 post vector delivery. However, two of the five macaques administered with AAVhu37.E03.TTR were free of a detectable antibody response for 30 weeks post vector administration. Overall, the study supports the continued development of AAV-based gene therapeutics for hemophilia A using the AAVhu37 capsid.
Keywords: adeno-associated virus; factor VIII; hemophilia A; monkey.
Figures



References
-
- Rangarajan S, Walsh L, Lester W, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med 2017;377:2519–2530. - PubMed
-
- Sullivan SK, George LA, Ragni MV, et al. SPK-8011: preliminary results from a Phase 1/2 trial of investigational gene therapy for hemophilia confirm transgene derived increases in FVIII activity that are persistent and stable beyond eight months. Mol Ther 2018;26:163.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

