Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy
- PMID: 29891009
- PMCID: PMC5996496
- DOI: 10.1186/s40425-018-0371-5
Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy
Abstract
Background: Corticosteroids are routinely utilized to alleviate edema in patients with intracranial lesions and are first-line agents to combat immune-related adverse events (irAEs) that arise with immune checkpoint blockade treatment. However, it is not known if or when corticosteroids can be administered without abrogating the efforts of immunotherapy. The purpose of this study was to evaluate the impact of dexamethasone on lymphocyte activation and proliferation during checkpoint blockade to provide guidance for corticosteroid use while immunotherapy is being implemented as a cancer treatment.
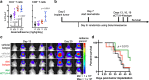
Methods: Lymphocyte proliferation, differentiation, and cytokine production were evaluated during dexamethasone exposure. Human T cells were stimulated through CD3 ligation and co-stimulated either directly by CD28 ligation or by providing CD80, a shared ligand for CD28 and CTLA-4. CTLA-4 signaling was inhibited by antibody blockade using ipilimumab which has been approved for the treatment of several solid tumors. The in vivo effects of dexamethasone during checkpoint blockade were evaluated using the GL261 syngeneic mouse intracranial model, and immune populations were profiled by flow cytometry.
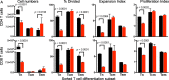
Results: Dexamethasone upregulated CTLA-4 mRNA and protein in CD4 and CD8 T cells and blocked CD28-mediated cell cycle entry and differentiation. Naïve T cells were most sensitive, leading to a decrease of the development of more differentiated subsets. Resistance to dexamethasone was conferred by blocking CTLA-4 or providing strong CD28 co-stimulation prior to dexamethasone exposure. CTLA-4 blockade increased IFNγ expression, but not IL-2, in stimulated human peripheral blood T cells exposed to dexamethasone. Finally, we found that CTLA-4 blockade partially rescued T cell numbers in mice bearing intracranial gliomas. CTLA-4 blockade was associated with increased IFNγ-producing tumor-infiltrating T cells and extended survival of dexamethasone-treated mice.
Conclusions: Dexamethasone-mediated T cell suppression diminishes naïve T cell proliferation and differentiation by attenuating the CD28 co-stimulatory pathway. However, CTLA-4, but not PD-1 blockade can partially prevent some of the inhibitory effects of dexamethasone on the immune response.
Keywords: Checkpoint blockade; Corticosteroids; Dexamethasone; Glioma; Immunotherapy.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable; not a clinical trial.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
