An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for a cluster randomised controlled feasibility trial
- PMID: 29891011
- PMCID: PMC5996505
- DOI: 10.1186/s13063-018-2669-5
An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for a cluster randomised controlled feasibility trial
Abstract
Background: Despite the evidence that many stroke survivors report longer term unmet needs, the provision of longer term care is limited. To address this, we are conducting a programme of research to develop an evidence-based and replicable longer term care strategy. The developed complex intervention (named New Start), which includes needs identification, exploration of social networks and components of problem solving and self-management, was designed to improve quality of life by addressing unmet needs and increasing participation.
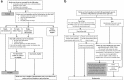
Methods/design: A multicentre, cluster randomised controlled feasibility trial designed to inform the design of a possible future definitive cluster randomised controlled trial (cRCT) and explore the potential clinical and cost-effectiveness of New Start. Ten stroke services across the UK will be randomised on a 1:1 basis either to implement New Start or continue with usual care only. New Start will be delivered by trained facilitators and will be offered to all stroke survivors within the services allocated to the intervention arm. Stroke survivors will be eligible for the trial if they are 4-6 months post-stroke and residing in the community. Carers (if available) will also be invited to take part. Invitation to participate will be initiated by post and outcome measures will be collected via postal questionnaires at 3, 6 and 9 months after recruitment. Outcome data relating to perceived health and disability, wellbeing and quality of life as well as unmet needs will be collected. A 'study within a trial' (SWAT) is planned to determine the most acceptable format in which to provide the postal questionnaires. Details of health and social care service usage will also be collected to inform the economic evaluation. The feasibility of recruiting services and stroke survivors to the trial and of collecting postal outcomes will be assessed and the potential for effectiveness will be investigated. An embedded process evaluation (reported separately) will assess implementation fidelity and explore and clarify causal assumptions regarding implementation.
Discussion: This feasibility trial with embedded process evaluation will allow us to gather important and detailed data regarding methodological and implementation issues to inform the design of a possible future definitive cRCT of this complex intervention.
Trial registration: ISRCTN38920246 . Registered 22 June 2016.
Keywords: Cluster trial; Community; Complex intervention; Facilitated self-management; Feasibility trial; Longer term; Stroke; Study within a trial (SWAT).
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval has been obtained through the Yorkshire & The Humber - Leeds East Research Ethics Committee (REC) (Ref: 16/YH/0068). Ethical approval includes agreed processes for obtaining consent (or assent) from (or on behalf of) all stroke survivors and carers with and without capacity, in line with the provisions of the Mental Capacity Act. Informed consent (or consultee declaration) will be obtained from all participants in the study.
Any protocol amendments will be approved by the REC prior to informing researchers and participating sites of any related process changes. Amendments impacting participants’ involvement will be communicated to them in an appropriate manner, as agreed by the REC.
At the time of writing, the current approved protocol is version 6, dated 18th July 2017.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
An intervention to support stroke survivors and their carers in the longer term: results of a cluster randomised controlled feasibility trial (LoTS2Care).Pilot Feasibility Stud. 2023 Mar 15;9(1):40. doi: 10.1186/s40814-023-01258-6. Pilot Feasibility Stud. 2023. PMID: 36922866 Free PMC article.
-
Longer-term health and social care strategies for stroke survivors and their carers: the LoTS2Care research programme including cluster feasibility RCT.Southampton (UK): NIHR Journals Library; 2021 Mar. Southampton (UK): NIHR Journals Library; 2021 Mar. PMID: 33819000 Free Books & Documents. Review.
-
An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for the process evaluation of a cluster randomised controlled feasibility trial.Trials. 2018 Jul 11;19(1):368. doi: 10.1186/s13063-018-2683-7. Trials. 2018. PMID: 29996895 Free PMC article.
-
An extended stroke rehabilitation service for people who have had a stroke: the EXTRAS RCT.Health Technol Assess. 2020 May;24(24):1-202. doi: 10.3310/hta24240. Health Technol Assess. 2020. PMID: 32468989 Free PMC article. Clinical Trial.
-
Development and evaluation of tools and an intervention to improve patient- and carer-centred outcomes in Longer-Term Stroke care and exploration of adjustment post stroke: the LoTS care research programme.Southampton (UK): NIHR Journals Library; 2014 Dec. Southampton (UK): NIHR Journals Library; 2014 Dec. PMID: 25642527 Free Books & Documents. Review.
Cited by
-
An intervention to support stroke survivors and their carers in the longer term: results of a cluster randomised controlled feasibility trial (LoTS2Care).Pilot Feasibility Stud. 2023 Mar 15;9(1):40. doi: 10.1186/s40814-023-01258-6. Pilot Feasibility Stud. 2023. PMID: 36922866 Free PMC article.
-
Supporting carers of stroke survivors to reduce carer burden: development of the Preparing is Caring intervention using Intervention Mapping.BMC Public Health. 2019 Oct 29;19(1):1408. doi: 10.1186/s12889-019-7615-2. BMC Public Health. 2019. PMID: 31664985 Free PMC article.
-
Development of a self-management intervention for stroke survivors with aphasia using co-production and behaviour change theory: An outline of methods and processes.PLoS One. 2021 Nov 23;16(11):e0259103. doi: 10.1371/journal.pone.0259103. eCollection 2021. PLoS One. 2021. PMID: 34813602 Free PMC article.
-
Patients' experience of and participation in a stroke self-management programme, My Life After Stroke (MLAS): a multimethod study.BMJ Open. 2022 Nov 15;12(11):e062700. doi: 10.1136/bmjopen-2022-062700. BMJ Open. 2022. PMID: 36379661 Free PMC article. Clinical Trial.
-
Effectiveness of a smartphone-enabled dyadic self-care programme (SDSCP) for stroke survivors and caregivers: study protocol for a randomised controlled trial.BMJ Open. 2023 Sep 4;13(9):e073016. doi: 10.1136/bmjopen-2023-073016. BMJ Open. 2023. PMID: 37666544 Free PMC article.
References
-
- Stroke Association. State of the Nation: Stroke Statistics. 2017. https://www.stroke.org.uk/resources/state-nation-stroke-statistics.
-
- Murray J, Young J, Forster A. Review of longer-term problems after a disabling stroke. Rev Clin Gerontol. 2007;17(4):277–292. doi: 10.1017/S0959259808002608. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

