Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain
- PMID: 29891013
- PMCID: PMC5996526
- DOI: 10.1186/s40478-018-0552-y
Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain
Abstract
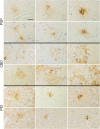
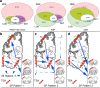
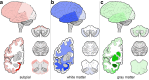
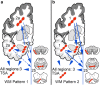
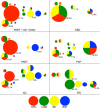
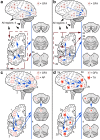
Aging-related tau astrogliopathy (ARTAG) describes tau pathology in astrocytes in different locations and anatomical regions. In the present study we addressed the question of whether sequential distribution patterns can be recognized for ARTAG or astroglial tau pathologies in both primary FTLD-tauopathies and non-FTLD-tauopathy cases. By evaluating 687 postmortem brains with diverse disorders we identified ARTAG in 455. We evaluated frequencies and hierarchical clustering of anatomical involvement and used conditional probability and logistic regression to model the sequential distribution of ARTAG and astroglial tau pathologies across different brain regions. For subpial and white matter ARTAG we recognize three and two patterns, respectively, each with three stages initiated or ending in the amygdala. Subependymal ARTAG does not show a clear sequential pattern. For grey matter (GM) ARTAG we recognize four stages including a striatal pathway of spreading towards the cortex and/or amygdala, and the brainstem, and an amygdala pathway, which precedes the involvement of the striatum and/or cortex and proceeds towards the brainstem. GM ARTAG and astrocytic plaque pathology in corticobasal degeneration follows a predominantly frontal-parietal cortical to temporal-occipital cortical, to subcortical, to brainstem pathway (four stages). GM ARTAG and tufted astrocyte pathology in progressive supranuclear palsy shows a striatum to frontal-parietal cortical to temporal to occipital, to amygdala, and to brainstem sequence (four stages). In Pick's disease cases with astroglial tau pathology an overlapping pattern with PSP can be appreciated. We conclude that tau-astrogliopathy type-specific sequential patterns cannot be simplified as neuron-based staging systems. The proposed cytopathological and hierarchical stages provide a conceptual approach to identify the initial steps of the pathogenesis of tau pathologies in ARTAG and primary FTLD-tauopathies.
Keywords: ARTAG; Aging-related tau astrogliopathy; Astrocytic plaque; Brain barrier; Hierarchical involvement; Ramified astrocyte; Spreading; Tau; Tauopathy; Tufted astrocyte.
Conflict of interest statement
Ethics approval and consent to participate
Informed consent was obtained from next of kin in accordance with institutional review board guidelines of the University of Pennsylvania.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

