Uncoupling ITIM receptor G6b-B from tyrosine phosphatases Shp1 and Shp2 disrupts murine platelet homeostasis
- PMID: 29891536
- PMCID: PMC6212653
- DOI: 10.1182/blood-2017-10-802975
Uncoupling ITIM receptor G6b-B from tyrosine phosphatases Shp1 and Shp2 disrupts murine platelet homeostasis
Abstract
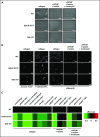
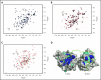
The immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing receptor G6b-B has emerged as a key regulator of platelet homeostasis. However, it remains unclear how it mediates its effects. Tyrosine phosphorylation of ITIM and immunoreceptor tyrosine-based switch motif (ITSM) within the cytoplasmic tail of G6b-B provides a docking site for Src homology 2 domain-containing protein-tyrosine phosphatases Shp1 and Shp2, which are also critical regulators of platelet production and function. In this study, we investigate the physiological consequences of uncoupling G6b-B from Shp1 and Shp2. To address this, we generated a transgenic mouse model expressing a mutant form of G6b-B in which tyrosine residues 212 and 238 within ITIM and ITSM were mutated to phenylalanine. Mice homozygous for the mutation (G6b-B diY/F) were macrothrombocytopenic, as a result of the reduction in platelet production, and had large clusters of megakaryocytes and myelofibrosis at sites of hematopoiesis, similar to those observed in G6b-deficient mice and patients. Platelets from G6b-B diY/F mice were hyporesponsive to collagen, as a result of the significant reduction in the expression of the immunoreceptor tyrosine-based activation motif (ITAM)-containing collagen receptor complex GPVI-FcR γ-chain, as well as thrombin, which could be partially rescued by costimulating the platelets with adenosine diphosphate. In contrast, platelets from G6b-B diY/F, G6b KO, and megakaryocyte-specific Shp2 KO mice were hyperresponsive to antibody-mediated cross-linking of the hemi-ITAM-containing podoplanin receptor CLEC-2, suggesting that G6b-B inhibits CLEC-2-mediated platelet activation through Shp2. Findings from this study demonstrate that G6b-B must engage with Shp1 and Shp2 to mediate its regulatory effects on platelet homeostasis.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

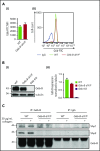
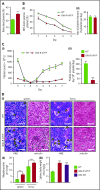
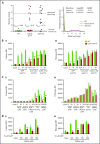
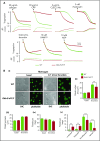

Comment in
-
G6b-B: the "Y's" and wherefores.Blood. 2018 Sep 27;132(13):1359-1360. doi: 10.1182/blood-2018-06-858738. Blood. 2018. PMID: 30262581 Free PMC article.
References
-
- George JN. Platelets. Lancet. 2000;355(9214):1531-1539. - PubMed
-
- Hartwig JH. The platelet: form and function. Semin Hematol. 2006;43(1 suppl 1):S94-S100. - PubMed
-
- Daëron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224(1):11-43. - PubMed
-
- Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29(1):665-705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

