Translational Control in Virus-Infected Cells
- PMID: 29891561
- PMCID: PMC6396331
- DOI: 10.1101/cshperspect.a033001
Translational Control in Virus-Infected Cells
Abstract
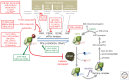
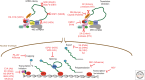
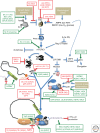
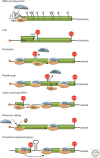
As obligate intracellular parasites, virus reproduction requires host cell functions. Despite variations in genome size and configuration, nucleic acid composition, and their repertoire of encoded functions, all viruses remain unconditionally dependent on the protein synthesis machinery resident within their cellular hosts to translate viral messenger RNAs (mRNAs). A complex signaling network responsive to physiological stress, including infection, regulates host translation factors and ribosome availability. Furthermore, access to the translation apparatus is patrolled by powerful host immune defenses programmed to restrict viral invaders. Here, we review the tactics and mechanisms used by viruses to appropriate control over host ribosomes, subvert host defenses, and dominate the infected cell translational landscape. These not only define aspects of infection biology paramount for virus reproduction, but continue to drive fundamental discoveries into how cellular protein synthesis is controlled in health and disease.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Abbas YM, Laudenbach BT, Martinez-Montero S, Cencic R, Habjan M, Pichlmair A, Damha MJ, Pelletier J, Nagar B. 2017. Structure of human IFIT1 with capped RNA reveals adaptable mRNA binding and mechanisms for sensing N1 and N2 ribose 2′-O methylations. Proc Natl Acad Sci 114: E2106–E2115. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
