Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses
- PMID: 29891600
- PMCID: PMC6105843
- DOI: 10.1128/AAC.00766-18
Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses
Abstract
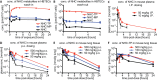
Morbidity and mortality resulting from influenza-like disease are a threat, especially for older adults. To improve case management, next-generation broad-spectrum antiviral therapeutics that are efficacious against major drivers of influenza-like disease, including influenza viruses and respiratory syncytial virus (RSV), are urgently needed. Using a dual-pathogen high-throughput screening protocol for influenza A virus (IAV) and RSV inhibitors, we have identified N4-hydroxycytidine (NHC) as a potent inhibitor of RSV, influenza B viruses, and IAVs of human, avian, and swine origins. Biochemical in vitro polymerase assays and viral RNA sequencing revealed that the ribonucleotide analog is incorporated into nascent viral RNAs in place of cytidine, increasing the frequency of viral mutagenesis. Viral passaging in cell culture in the presence of an inhibitor did not induce robust resistance. Pharmacokinetic profiling demonstrated dose-dependent oral bioavailability of 36 to 56%, sustained levels of the active 5'-triphosphate anabolite in primary human airway cells and mouse lung tissue, and good tolerability after extended dosing at 800 mg/kg of body weight/day. The compound was orally efficacious against RSV and both seasonal and highly pathogenic avian IAVs in mouse models, reducing lung virus loads and alleviating disease biomarkers. Oral dosing reduced IAV burdens in a guinea pig transmission model and suppressed virus spread to uninfected contact animals through direct transmission. Based on its broad-spectrum efficacy and pharmacokinetic properties, NHC is a promising candidate for future clinical development as a treatment option for influenza-like diseases.
Keywords: antiviral agents; influenza; nucleoside analogs; respiratory syncytial virus.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- Skowronski DM, Chambers C, De Serres G, Dickinson JA, Winter A-L, Hickman R, Chan T, Jassem AN, Drews SJ, Charest H, Gubbay JB, Bastien N, Li Y, Krajden M. 2018. Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Euro Surveill 23:pii=18-00035. doi:10.2807/1560-7917.ES.2018.23.5.18-00035. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

