Molecular Confirmation of the Linkage between the Rhizopus oryzae CYP51A Gene Coding Region and Its Intrinsic Voriconazole and Fluconazole Resistance
- PMID: 29891608
- PMCID: PMC6105841
- DOI: 10.1128/AAC.00224-18
Molecular Confirmation of the Linkage between the Rhizopus oryzae CYP51A Gene Coding Region and Its Intrinsic Voriconazole and Fluconazole Resistance
Abstract
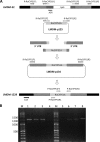
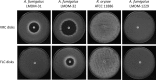
Rhizopus oryzae is the most prevalent causative agent of mucormycosis, an increasingly reported opportunistic fungal infection. These Mucorales are intrinsically resistant to Candida- and Aspergillus-active antifungal azole drugs, such as fluconazole (FLC) and voriconazole, respectively. Despite its importance, the molecular mechanisms of its intrinsic azole resistance have not been elucidated yet. The aim of this work was to establish if the Rhizopus oryzaeCYP51 genes are uniquely responsible for intrinsic voriconazole and fluconazole resistance in these fungal pathogens. Two CYP51 genes were identified in the R. oryzae genome. We classified them as CYP51A and CYP51B based on their sequence similarity with other known fungal CYP51 genes. Later, we obtained a chimeric Aspergillus fumigatus strain harboring a functional R. oryzae CYP51A gene expressed under the regulation of the wild-type A. fumigatusCYP51A promoter and terminator. The mutant was selected after transformation by using a novel procedure taking advantage of the FLC hypersusceptibility of the A. fumigatusCYP51A deletion mutant used as the recipient strain. The azole susceptibility patterns of the A. fumigatus transformants harboring R. oryzae CYP51A mimicked exactly the azole susceptibility patterns of this mucormycete. The data presented in this work demonstrate that the R. oryzae CYP51A coding sequence is uniquely responsible for the R. oryzae azole susceptibility patterns.
Keywords: CYP51; Mucorales; Rhizopus; azole; fluconazole; intrinsic resistance; molecular mechanism; resistance; voriconazole.
Copyright © 2018 American Society for Microbiology.
Figures

References
-
- Kontoyiannis DP, Lionakis MS, Lewis RE, Chamilos G, Healy M, Perego C, Safdar A, Kantarjian H, Champlin R, Walsh TJ, Raad II. 2005. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis 191:1350–1360. doi: 10.1086/428780. - DOI - PubMed
-
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. - DOI - PubMed
-
- Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, B DePauw, Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group . 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415. doi: 10.1056/NEJMoa020191. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

