Severe Renal Impairment Has Minimal Impact on Doravirine Pharmacokinetics
- PMID: 29891610
- PMCID: PMC6105803
- DOI: 10.1128/AAC.00326-18
Severe Renal Impairment Has Minimal Impact on Doravirine Pharmacokinetics
Abstract
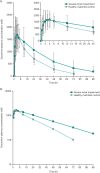
Doravirine is a novel nonnucleoside reverse transcriptase inhibitor in development for use with other antiretroviral therapies to treat human immunodeficiency virus type 1 (HIV-1) infection. Doravirine metabolism predominantly occurs via cytochrome P450 3A with <10% of elimination occurring via the renal pathway. As severe renal impairment can alter the pharmacokinetics (PK) of metabolically eliminated drugs, the effect of severe renal impairment on doravirine PK was assessed. A single dose of doravirine 100 mg was administered to subjects aged 18 to 75 years with an estimated glomerular filtration rate (eGFR) of <30 ml/min/1.73 m2 (severe renal impairment group) and healthy controls with an eGFR of ≥80 ml/min/1.73 m2, matched to the mean of the renal impairment group by age (±10 years) and weight (±10 kg). Doravirine plasma concentrations were determined at regular intervals, and safety was monitored throughout. The geometric mean ratios (90% confidence interval) for severe renal impairment/healthy subjects were 1.43 (1.00, 2.04), 1.38 (0.99, 1.92), and 0.83 (0.61, 1.15) for the plasma doravirine area under the curve from zero to infinity (AUC0-∞), plasma concentration at 24 h postdose (C24), and maximum plasma concentration (Cmax), respectively. Doravirine was generally well tolerated in both groups. Based on the overall efficacy, safety, and PK profile of doravirine, the minor effect of severe renal impairment on doravirine PK observed in this study is not considered clinically meaningful. (This study has been registered at ClinicalTrials.gov under identifier NCT02641067.).
Keywords: HIV; doravirine; pharmacokinetics; renal impairment.
Copyright © 2018 American Society for Microbiology.
Figures

References
-
- Feng M, Wang D, Grobler JA, Hazuda DJ, Miller MD, Lai M-T. 2015. In vitro resistance selection with doravirine (MK-1439), a novel nonnucleoside reverse transcriptase inhibitor with distinct mutation development pathways. Antimicrob Agents Chemother 59:590–598. doi: 10.1128/AAC.04201-14. - DOI - PMC - PubMed
-
- Feng M, Sachs NA, Xu M, Grobler J, Blair W, Hazuda DJ, Miller MD, Lai M-T. 2016. Doravirine suppresses common nonnucleoside reverse transcriptase inhibitor-associated mutants at clinically relevant concentrations. Antimicrob Agents Chemother 60:2241–2247. doi: 10.1128/AAC.02650-15. - DOI - PMC - PubMed
-
- Squires KE, Molina J-M, Sax PE, Wong W-W, Orkin C, Sussmann O, Kaplan R, Lupinacci L, Rodgers A, Xu X, Lin G, Kumar S, Sklar P, Nguyen B-Y, Hanna GJ, Hwang C, Martin E, DRIVE-AHEAD Study Team . 2017. Fixed dose combination of doravirine/lamivudine/TDF is non-inferior to efavirenz/emtricitabine/TDF in treatment-naïve adults with HIV-1 infection: week 48 results of the phase 3 DRIVE-AHEAD study, abstr TUAB0104LB. International AIDS Society (IAS), July 23 to 26, Paris, France.
-
- Molina JM, Squires K, Sax PE, Cahn P, Lombaard J, DeJesus E, Lai MT, Xu X, Rodgers A, Lupinacci L, Kumar S, Sklar P, Nguyen BY, Hanna GJ, Hwang C, DRIVE-FORWARD Study Group . 2018. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV 5:e211–e220. doi: 10.1016/S2352-3018(18)30021-3. - DOI - PubMed
-
- Orkin C, Squires KE, Molina J-M, Sax PE, Wong W-W, Sussmann O, Kaplan R, Rodgers A, Xu X, Lin G, Kumar S, Hanna GJ, Hwang C, Martin E, DRIVE-AHEAD Study Team . 2018. Similar efficacy and safety by subgroup in DRIVE-AHEAD: DOR/3TC/TDF vs EFV/FTC/TDF, poster number 491. CROI 2018, March 4 to 7, Boston, MA.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

