Differentiated human airway organoids to assess infectivity of emerging influenza virus
- PMID: 29891677
- PMCID: PMC6042130
- DOI: 10.1073/pnas.1806308115
Differentiated human airway organoids to assess infectivity of emerging influenza virus
Abstract
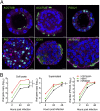
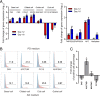
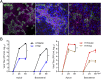
Novel reassortant avian influenza H7N9 virus and pandemic 2009 H1N1 (H1N1pdm) virus cause human infections, while avian H7N2 and swine H1N1 virus mainly infect birds and pigs, respectively. There is no robust in vitro model for assessing the infectivity of emerging viruses in humans. Based on a recently established method, we generated long-term expanding 3D human airway organoids which accommodate four types of airway epithelial cells: ciliated, goblet, club, and basal cells. We report differentiation conditions which increase ciliated cell numbers to a nearly physiological level with synchronously beating cilia readily discernible in every organoid. In addition, the differentiation conditions induce elevated levels of serine proteases, which are essential for productive infection of human influenza viruses and low-pathogenic avian influenza viruses. We also established improved 2D monolayer culture conditions for the differentiated airway organoids. To demonstrate the ability of differentiated airway organoids to identify human-infective virus, 3D and 2D differentiated airway organoids are applied to evaluate two pairs of viruses with known distinct infectivity in humans, H7N9/Ah versus H7N2 and H1N1pdm versus an H1N1 strain isolated from swine (H1N1sw). The human-infective H7N9/Ah virus replicated more robustly than the poorly human-infective H7N2 virus; the highly human-infective H1N1pdm virus replicated to a higher titer than the counterpart H1N1sw. Collectively, we developed differentiated human airway organoids which can morphologically and functionally simulate human airway epithelium. These differentiated airway organoids can be applied for rapid assessment of the infectivity of emerging respiratory viruses to human.
Keywords: airway organoid; infectivity; influenza virus; proximal differentiation.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Klenk HD. Influenza viruses en route from birds to man. Cell Host Microbe. 2014;15:653–654. - PubMed
-
- World Health Organization 2017 Human infection with avian influenza A(H7N9) virus China. Available at www.who.int/csr/don/26-october-2017-ah7n9-china/en/WHO. Accessed May 30, 2018.
-
- Dawood FS, et al. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

